Polypropylene glycol
Polypropylene glycol or polypropylene oxide is the polymer of propylene glycol. Chemically it is a polyether, and, more generally speaking, it's a polyalkylene glycol (PAG). The term polypropylene glycol or PPG is reserved for low to medium range molar mass polymer when the nature of the end-group, which is usually a hydroxyl group, still matters. The term "oxide" is used for high molar mass polymer when end-groups no longer affect polymer properties. In 2003, 60% of the annual production of propylene oxide of 6.6×106 tonnes was converted into the polymer.[1]
Polymerization
Polypropylene glycol is produced by ring-opening polymerization of propylene oxide. The initiator is an alcohol and the catalyst a base, usually potassium hydroxide. When the initiator is ethylene glycol or water the polymer is linear. With a multifunctional initiator like glycerine, pentaerythritol or sorbitol the polymer branches out.
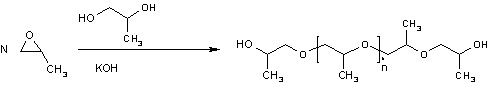
Conventional polymerization of propylene oxide results in an atactic polymer. The isotactic polymer can be produced from optically active propylene oxide, but at a high cost. A salen cobalt catalyst was reported in 2005 to provide isotactic polymerization of the prochiral propylene oxide[2]
Properties
PPG has many properties in common with polyethylene glycol. The polymer is a liquid at room temperature. Solubility in water decreases rapidly with increasing molar mass. Secondary hydroxyl groups in PPG are less reactive than primary hydroxyl groups in polyethylene glycol. PPG is less toxic than PEG, so biotechnologicals are now produced in PPG.

Uses
PPG is used in many formulations for polyurethanes. It is used as a rheology modifier.
PPG is used as a surfactant, wetting agent, dispersant in leather finishing.
PPG is also employed as a tuning reference and calibrant in mass spectrometry.
PPG is used as a primary ingredient in the making of paintballs.
PPG is used as a primary ingredient in the making of some laxatives[3].
References
- A Highly Active, Isospecific Cobalt Catalyst for Propylene Oxide Polymerization Kathryn L. Peretti, Hiroharu Ajiro, Claire T. Cohen, Emil B. Lobkovsky, and Geoffrey W. Coates J. Am. Chem. Soc., 127 (33), 11566 -11567, 2005. Abstract
- A Highly Active, Isospecific Cobalt Catalyst for Propylene Oxide Polymerization Kathryn L. Peretti, Hiroharu Ajiro, Claire T. Cohen, Emil B. Lobkovsky, and Geoffrey W. Coates J. Am. Chem. Soc., 127 (33), 11566 -11567, 2005. Abstract
- Miralax label