Bisulfite
The bisulfite ion (IUPAC-recommended nomenclature: hydrogen sulfite) is the ion HSO3−. Salts containing the HSO3− ion are termed bisulfites also known as "sulfite lyes".[1]
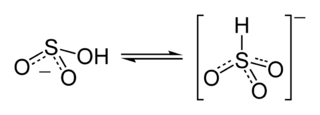
Structure
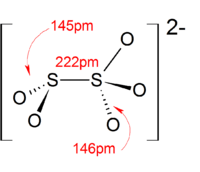
The proton in bisulfite shuttles between S and O. The H-S tautomer has C3v symmetry. The H-O tautomer has been observed by 17O NMR spectroscopy.[1][2] The C3v structure is supported by X-ray crystallography and, in aqueous solution, by Raman spectroscopy (ν(S–H) = 2500 cm−1).
Reactions
Acid-base reactions
Solutions of bisulfite are typically prepared by treatment of sulfur dioxide with aqueous base:[3]
- SO2 + OH− → HSO3−
HSO3− is the conjugate base of sulfurous acid, (H2SO3), which does not exist in the aqueous phase. An equilibrium that is much more consistent with spectroscopic evidence is:
- SO2 + H2O ⇌ HSO3− + H+
HSO3− is a weak acidic species with a pKa of 6.97. Its conjugate base is the sulfite ion, SO32−:
- HSO3− ⇌ SO32− + H+
Dehydration/hydration equilibria
Attempted isolation of the common salts of bisulfite results in dehydration of the anion with formation of metabisulfite (S
2O2−
5), also known as disulfite:
- 2 HSO3− ⇌ S2O52− + H2O
Because of this equilibrium, anhydrous sodium and potassium salts of bisulfite cannot be obtained. However, there are some reports of anhydrous bisulfites with large counter ions.[4]
Redox reactions
Bisulfite is a good reducing agent, especially for oxygen scrubbing:
- 2 HSO3− + O2 → 2 SO42− + 2 H+
Its reducing properties are exploited to precipitate gold from auric acid (gold dissolved in aqua regia) and reduce hexavalent chromium to trivalent chromium. In water chlorination, sodium bisulfite is used to reduce the residual 'chlorine' which can have a negative impact on aquatic life.
Organic synthesis
In organic chemistry, "sodium bisulfite" is a routine reagent but its composition is imprecisely known. Sodium bisulfite is used interchangeable with sodium metabisulfite, which is Na2S2O5 as a solid but dissolves to give a solution of Na+HSO3−.
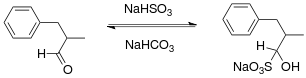
Bisulfite forms adducts with aldehyde and with certain cyclic ketones to give α-hydroxysulfonic acids.[6] This reaction is useful for the purification of aldehydes. The bisulfite adducts precipitate as solids from solution. The reaction can be reversed in base.[7] Examples of such procedures are described for benzaldehyde,[8] 2-tetralone,[9] citral,[10] the ethyl ester of pyruvic acid[11] and glyoxal.[12] In the ring-expansion reaction of cyclohexanone with diazald, the bisulfite reaction is reported to allow differentiation between the primary reaction product cycloheptanone and the main contaminant cyclooctanone.[13]
Another use of bisulfite in organic chemistry is as a mild reducing agent, e.g. to remove traces or excess amounts of chlorine, bromine, iodine, hypochlorite salts, osmate esters, chromium trioxide and potassium permanganate. Sodium bisulfite is a decoloration agent in purification procedures because it reduces strongly coloured oxidizing agents, conjugated alkenes and carbonyl compounds.
Bisulfite is also the key ingredient in the Bucherer reaction. In this reaction an aromatic hydroxyl group is converted to the corresponding amine group. This reaction is reversible reaction. The first step in this reaction is an addition reaction of sodium bisulfite to an aromatic double bond. The Bucherer carbazole synthesis is a related organic reaction that uses sodium bisulfite as a reagent.
Bisulfite DNA sequencing
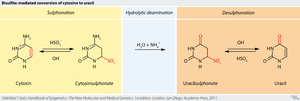
Sodium bisulfite is used in the analysis of the methylation status of cytosines in DNA.
In this technique, sodium bisulfite deaminates cytosine into uracil, but does not affect 5-methylcytosine, a methylated form of cytosine with a methyl group attached to carbon 5.
When the bisulfite-treated DNA is amplified via polymerase chain reaction, the uracil is amplified as thymine and the methylated cytosines are amplified as cytosine. DNA sequencing techniques are then used to read the sequence of the bisulfite-treated DNA. Those cytosines that are read as cytosines after sequencing represent methylated cytosines, while those that are read as thymines represent unmethylated cytosines in the genomic DNA.[14]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Horner, D. A.; Connick, R. E. (1986). "Equilibrium quotient for the isomerization of bisulfite ion from HSO3− to SO3H−". Inorganic Chemistry. 25 (14): 2414–2417. doi:10.1021/ic00234a026.
- Barberá, José Jiménez; Metzger, Adolf; Wolf, Manfred (2000). "Sulfites, Thiosulfates, and Dithionitesl Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477.
- Maylor, R.; Gill, J.B.; Goodall, D.C. (July 1971). "Some studies on anhydrous cobalt sulphite". Journal of Inorganic and Nuclear Chemistry. 33 (7): 1975–1979. doi:10.1016/0022-1902(71)80558-4.
- Carter, Kay L.; Siddiquee, Tasneem A.; Murphy, Kristen L.; Bennett, Dennis W. (18 March 2004). "The surprisingly elusive crystal structure of sodium metabisulfite". Acta Crystallographica Section B Structural Science. 60 (2): 155–162. doi:10.1107/S0108768104003325.
- Young, Steven D.; Buse, Charles T.; Heathcock, Clayton H. (1990). "2-Methyl-2-(Trimethylsiloxy)pentan-3-one". Organic Syntheses.; Collective Volume, 7, p. 381
- Buntin, S. A.; Heck, Richard F. (1990). "2-Methyl-3-phenylpropanal". Organic Syntheses.; Collective Volume, 7, p. 361
- Taylor, Harold M.; Hauser, Charles R. (1973). "α-(N,N-Dimethylamino)phenylacetonitrile". Organic Syntheses.; Collective Volume, 5, p. 437
- Soffer, M. D.; Bellis, M. P.; Gellerson, Hilda E.; Stewart, Roberta A. (1963). "β-Tetralone". Organic Syntheses.; Collective Volume, 4, p. 903
- Russell, Alfred; Kenyon, R. L. (1955). "Pseudoionone". Organic Syntheses.; Collective Volume, 3, p. 747
- Cornforth, J. W. (1963). "Ethyl Pyruvate". Organic Syntheses.; Collective Volume, 4, p. 467
- Ronzio, Anthony R.; Waugh, T. D. (1955). "Glyoxal Bisulfite". Organic Syntheses.; Collective Volume, 3, p. 438
- Dauben, Hyp J., Jr.; Ringold, Howard J.; Wade, Robert H.; Pearson, David L.; Anderson, Arthur G., Jr. "Cycloheptanone". Organic Syntheses. doi:10.15227/orgsyn.034.0019.; Collective Volume, 4, p. 221
- Frommer, M.; McDonald, L. E.; Millar, D. S.; Collis, C.M.; Watt, F.; Grigg, G.W.; Molloy P.L.; Paul, C.L. (1992). "A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands". PNAS. 89 (5): 1827–31. Bibcode:1992PNAS...89.1827F. doi:10.1073/pnas.89.5.1827. PMC 48546. PMID 1542678.