Disulfite
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion S
2O2−
5. It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the bisulfite HSO−
3 ion. These salts act equivalently to sodium bisulfite or potassium bisulfite.[2]
 | |
| Names | |
|---|---|
| IUPAC name
disulfite[1] | |
| Systematic IUPAC name
pentaoxido-1κ3O,2κ2O-disulfate(S—S)(2−)[1] | |
| Other names
metabisulfite ion pyrosulfite | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| S 2O2− 5 | |
| Conjugate acid | Disulfurous acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
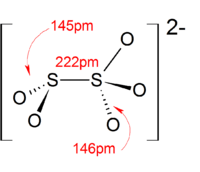
Structure
In contrast to disulfate (S
2O2−
7), disulfite ion (S
2O2−
5) has an unsymmetrical structure with an S-S bond. The oxidation state of the sulfur atom bonded to 3 oxygen atoms is +5 while oxidation number of other sulfur atom is +3.[3]
The anion consists of an SO2 group linked to an SO3 group, with the negative charge more localized on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å respectively.[4]
Production
Salts of disulfite ion are produced by dehydration of salts of bisulfite ion (HSO−
3). When solutions of sodium bisulfite or potassium bisulfite are evaporated, sodium metabisulfite and potassium metabisulfite result.[5]
- 2 HSO−
3
2O2−
5 + H2O
Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.[6]
Disulfite is the conjugate base of disulfurous acid (pyrosulfurous acid), which originates from sulfurous acid in accordance with the dehydration reaction above:
- 2 H2SO3 → 2 HSO−
3 + 2 H+ → H2S2O5 + H2O
The disulfite ion also arises from the addition of sulfur dioxide to the sulfite ion:
| HSO− 3 3 + H+ SO32− + SO2 2O2− 5 | 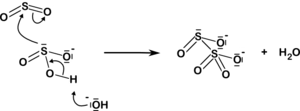 |
Use
Disulfites salts are used for preserving food and beverages.
Examples of disulphites
- Sodium metabisulphite (E223) and potassium metabisulphite (E224) are used as a preservative and antioxidant in food.
References
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- Johnstone, H. F. (1946). "Sulfites and Pyrosulfites of the Alkali Metals". Inorganic Syntheses. 2: 162–167. doi:10.1002/9780470132333.ch49. ISBN 9780470132333.
- Lindqvist, I.; Mörtsell, M. "The Structure of Potassium Pyrosulfite and the Nature of the Pyrosulfite Ion". Acta Crystallogr. (1957". 10: 406–409. doi:10.1107/S0365110X57001322. Cite journal requires
|journal=(help) - K. L. Carter, T. A. Siddiquee, K. L. Murphy, D. W. Bennett "The surprisingly elusive crystal structure of sodium metabisulfite" Acta Crystallogr. (2004). B60, 155–162. doi:10.1107/S0108768104003325
- Barberá, José Jiménez; Metzger, Adolf; Wolf, Manfred (2000). "Sulfites, Thiosulfates, and Dithionitesl Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477.
- Bassam Z. Shakhashiri: Chemical demonstrations: a handbook for teachers of chemistry The University of Wisconsin Press, 1992, p.9