Charles R. Hauser
Charles Roy Hauser (March 8, 1900 – January 6, 1970) was an American chemist. Hauser was a member of the National Academy of Sciences[1][2] and a professor of chemistry at Duke University.[3]
Charles R. Hauser | |
|---|---|
| Born | March 8, 1900 |
| Died | January 6, 1970 (aged 69) |
| Nationality | United States |
| Scientific career | |
| Fields | Chemistry |
Notable work
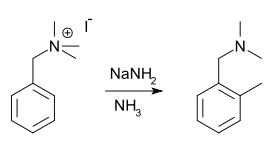
The Sommelet–Hauser rearrangement is a named reaction based on the work of Hauser[4] and Sommelet[5] involving the rearrangement of certain benzyl quaternary ammonium salts.[6][7] The reagent is sodium amide or another alkali metal amide and the reaction product a N,N-dialkylbenzylamine with a new alkyl group in the aromatic ortho position. For example, benzyltrimethylammonium iodide, [(C6H5CH2)N(CH3)3]I, rearranges in the presence of sodium amide to yield the o-methyl derivative of N,N-dimethylbenzylamine.[4]
Awards
His contributions were recognized by the following awards:[1]
- 1957 the Florida Section Award
- 1962 the Herty Medal
- 1967 the Medal for Synthetic Organic Chemistry from the Synthetic Organic Chemical Manufacturers' Association
References
- Bradsher, Charles K. Charles Roy Hauser. Biographical Memoirs, National Academy of Sciences. National Academies Press. Retrieved January 3, 2012.
- Science Academy Re-elects Bronk New York Times. April 30, 1958. Retrieved January 3, 2012.
- Inventory of the Charles Roy Hauser Papers University Archives, Duke University. Retrieved January 3, 2012.
- Kantor, S. W.; Hauser, C. R. (1951). "Rearrangements of Benzyltrimethylammonium Ion and Related Quaternary Ammonium Ions by Sodium Amide Involving Migration into the Ring". J. Am. Chem. Soc. 73 (9): 4122–4131. doi:10.1021/ja01153a022.
- M. Sommelet, Compt. Rend. 205, 56 (1937).
- March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- Organic Syntheses, Coll. Vol. 4, p.585 (1963); Vol. 34, p.61 (1954) Link.