Hydrohalogenation
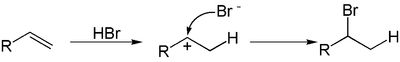
A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.[1][2][3]
If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with fewer hydrogen substituents, an observation known as Markovnikov's rule. This is due to the abstraction of a hydrogen atom by the alkene from the acid (HX) to form the most stable carbocation (relative stability: 3°>2°>1°>methyl), as well as generating a halogen anion.
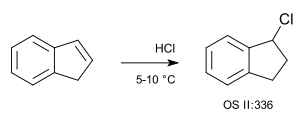
A simple example of a hydrochlorination is that of indene with hydrogen chloride gas (no solvent):[4]
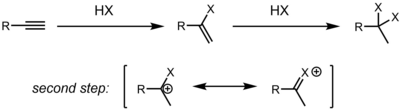
Alkynes also undergo hydrohalogenation reactions. Depending on the exact substrate, alkyne hydrohalogenation can proceed though a concerted protonation/nucleophilic attack (AdE3) or stepwise by first protonating the alkyne to form a vinyl cation, followed by attack of HX/X- to give the product (AdE2) (see electrophile for arrow pushing).[5] As in the case of alkenes, the regioselectivity is determined by the relative ability of the carbon atoms to stabilize positive charge (either a partial charge in the case of a concerted transition state or a full formal charge for a discrete vinyl cation). Depending on reaction conditions, the main product could be this initially formed alkenyl halide, or the product of twice hydrohalogenation to form a dihaloalkane. In most cases, the main regioisomer formed is the gem-dihaloalkane.[6] This regioselectivity is rationalized by the resonance stabilization of a neighboring carbocation by a lone pair on the initially installed halogen. Depending on relative rates of the two steps, it may be difficult to stop at the first stage, and often, mixtures of the mono and bis hydrohalogenation products are obtained.
Anti-Markovnikov addition
In the presence of peroxides, HBr adds to a given alkene in an anti-Markovnikov addition fashion.[7] This regiochemistry follows from the reaction mechanism, which favors formation of the most stable carbon radical intermediate (relative stability: 3° > 2° > 1°> methyl). The mechanism for this reaction is similar to a chain reaction such as free radical halogenation in which the peroxide promotes the formation of the bromide radical. Therefore, in the presence of peroxides, HBr adds so that the bromine atom is added to the carbon bearing the most numerous hydrogen substituents and hydrogen atoms will add to carbons bearing fewest hydrogen substituents. However, this process is restricted to addition of HBr.
Other hydrogen halides (HF, HCl, HI) do not behave in the manner described above.[8] The resulting 1-bromoalkanes are versatile alkylating agents. By reaction with dimethyl amine, they are precursors to fatty tertiary amines. By reaction with tertiary amines, long-chain alkyl bromides such as 1-bromododecane, give quaternary ammonium salts, which are used as phase transfer catalysts.[9]
With Michael acceptors the addition is also anti-Markovnikov because now a nucleophilic X− reacts in a nucleophilic conjugate addition for example in the reaction of HCl with acrolein.[10]
Scope
Recent research has found that adding silica gel or alumina to H-Cl (or H-Br) in dichloromethane increases the rate of reaction making it an easy one to carry out.
References
- Solomons, T.W. Graham; Fryhle, Craig B. (2003), Organic Chemistry (8th ed.), Wiley, ISBN 0-471-41799-8
- Smith, Janice G. (2007), Organic Chemistry (2nd ed.), McGraw-Hill, ISBN 0-07-332749-2
- P.J. Kropp; K.A. Dans; S.D. Crawford; M.W. Tubergen; K.D. Kepler; S.L Craig; V.P. Wilson (1990), "Surface-mediated reactions. 1. Hydrohalogenation of alkenes and alkynes", J. Am. Chem. Soc., 112 (112): 7433–7434, doi:10.1021/ja00176a075.
- R. A. Pacaud and C. F. H. Allen. "α-Hydroindone". Organic Syntheses.; Collective Volume, 2, p. 336
- Lowry, Thomas H. (1987). Mechanism and theory in organic chemistry. Richardson, Kathleen Schueller. (3rd ed.). New York: Harper & Row. ISBN 0-06-044084-8. OCLC 14214254.
- Vollhardt, K. Peter C.,. Organic chemistry : structure and function. Schore, Neil Eric, 1948- (Seventh ed.). New York, NY. ISBN 978-1-4641-2027-5. OCLC 866584251.CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link)
- March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. ISBN 0-471-85472-7
- The hydrogen-fluorine bond is simply too strong and therefore no fluorine radicals can be generated in the propagation step. Hydrogen chloride reacts too slowly, again reflecting the strength of the hydrogen-chlorine bond. Due to the weakness of the carbon-iodine bond necessary to complete the first step of the propagation phase, insufficient heat is released to proceed through the reaction successfully.
- Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. (2012). "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.CS1 maint: multiple names: authors list (link)
- C. Moureu and R. Chaux (1941). "β-Chloropropionic acid". Organic Syntheses.; Collective Volume, 1, p. 166