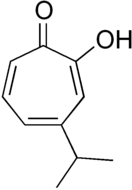
Hinokitiol
Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins.[2] Hinokitiol is widely used in oral care and treatment products.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Hydroxy-6-propan-2-ylcyclohepta-2,4,6-trien-1-one | |||
| Other names
β-Thujaplicin; 4-Isopropyltropolone | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.165 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H12O2 | |||
| Molar mass | 164.204 g·mol−1 | ||
| Appearance | Colorless to pale yellow crystals | ||
| Melting point | 50 to 52 °C (122 to 126 °F; 323 to 325 K) | ||
| Boiling point | 140 °C (284 °F; 413 K) at 10 mmHg | ||
| Hazards | |||
| Flash point | 140 °C (284 °F; 413 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hinolitiol was originally isolated in Taiwanese hinoki in 1936.[3] It is almost absent in Japanese hinoki while it is contained in high concentration (about 0.04% of heartwood mass) in Juniperus cedrus, hiba cedar wood (Thujopsis dolabrata) and western red cedar (Thuja plicata). It can be readily extracted from the cedarwood with solvent and ultrasonication.[4]
Hinokitiol is structurally related to tropolone, which lacks the isopropyl substituent. Tropolones are well-known chelating agents.
Antimicrobial activity
Hinokitiol has been shown to possess inhibitory effects on Chlamydia trachomatis and may be clinically useful as a topical drug.[5]
Products containing hinokitiol
Hinokitiol is used in a range of consumer products including cosmetics, toothpastes, oral sprays, sunscreens And hair-growth. In 2006, hinokitiol was categorised under the Domestic Substances List in Canada as non-persistent, non-bioaccumulative and non-toxic to aquatic organisms.[6]
In April 2020, Advance Nanotek, an Australian producer of zinc oxide, filed a joint patent application with AstiVita Limited, for an anti-viral composition that included oral care products.[7]
History
Hinokitiol was discovered in 1936 by Tetsuo Nozoe from the essential oil component of Taiwan cypress. The compound has a heptagonal molecular structure.
Research directions
In animals
Researchers screening a library of small biomolecules for signs of iron transport found that hinokitiol restored cell functionality. Further work by the team suggested a mechanism by which hinokitiol restores or reduces cell iron.[8] In mammals, they found that when rodents that had been engineered to lack "iron proteins" were fed hinokitiol, they regained iron uptake in the gut. In a similar study on zebrafish, the molecule restored hemoglobin production.[9]
References
- β-Thujaplicin at Sigma-Aldrich
- Chedgy RJ, Lim YW, Breuil C (May 2009). "Effects of leaching on fungal growth and decay of western redcedar". Canadian Journal of Microbiology. 55 (5): 578–86. doi:10.1139/W08-161. PMID 19483786.
- Murata I, Itô S, Asao T (December 2012). "Tetsuo Nozoe: chemistry and life". Chemical Record. 12 (6): 599–607. doi:10.1002/tcr.201200024. PMID 23242794.
- Chedgy RJ, Daniels CR, Kadla J, Breuil C (2007). "Screening fungi tolerant to Western red cedar (Thuja plicata Donn) extractives. Part 1. Mild extraction by ultrasonication and quantification of extractives by reverse-phase HPLC". Holzforschung. 61 (2): 190–194. doi:10.1515/HF.2007.033. S2CID 95994935.
- Chedgy R (2010). Secondary metabolites of Western red cedar (Thuja plicata): their biotechnological applications and role in conferring natural durability. LAP Lambert Academic Publishing. ISBN 978-3-8383-4661-8.
- Secretariat, Treasury Board of Canada; Secretariat, Treasury Board of Canada. "Detailed categorization results of the Domestic Substances List - Open Government Portal". open.canada.ca. Retrieved 2020-06-17.
- "IP Australia: AusPat". Australian Government - IP Australia. Retrieved 2020-05-20.
- Grillo AS, SantaMaria AM, Kafina MD, Cioffi AG, Huston NC, Han M, et al. (May 2017). "Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals". Science. 356 (6338): 608–616. Bibcode:2017Sci...356..608G. doi:10.1126/science.aah3862. PMC 5470741. PMID 28495746.
- Service RF (May 2017). "Iron Man molecule restores balance to cells". Science Magazine. AAAS. Retrieved 2020-05-20.