Haloketone
A haloketone in organic chemistry is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. The general structure is RR'C(X)C(=O)R where R is an alkyl or aryl residue and X any one of the halogens. The preferred conformation of a haloketone is that of a cisoid with the halogen and carbonyl sharing the same plane as the steric hindrance with the carbonyl alkyl group is generally larger.[1]
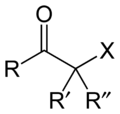
Haloketone synthesis
- Haloketones and halo carbonyl compounds in general are synthesized by reaction of carbonyl compounds with halogenation agents:
- Halogens, bromine and chlorine give monosubstitution, fluorine gives polysubstitution
- Tetrabutylammonium tribromide
- N-Bromosuccinimide
- 1,3-Dibromo-5,5-Dimethylhydantoin (DBDMH)
- In the Hell-Volhard-Zelinsky halogenation a carboxylic acid reacts with bromine in presence of phosphorus tribromide.
- In the Nierenstein reaction an acyl chloride reacts with diazomethane
Asymmetric synthesis
Efforts are reported in asymmetric synthesis of halocarbonyls through organocatalysis. In one study an acid chloride is converted into an α-halo-ester with a strong base (sodium hydride), a bromine donor and an organocatalyst based on proline and quinine:[2]
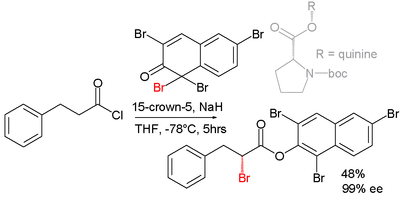
In the proposed reaction mechanism the base first converts the acid chloride to the ketene, the organocatalyst then introduces chirality through its quininoid tertiary amine, forming a ketene adduct.
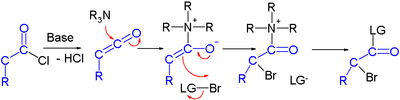
Haloketone reactions
Haloketones take part in several reaction types. In reaction with a nucleophile, two electrophilic sites are available and in reactions with a base several acidic protons exist due to the presence of two electron withdrawing groups. The carbon halogen bond experiences increases polarity from the inductive effect of the carbonyl group making the carbon atom more electropositive.
- In nucleophilic aliphatic substitution reactions with potassium iodide in acetone, 1-chloro-2-propanone reacts faster than n-propylchloride by a factor of 36 000.
- In the Favorskii rearrangement a base abstracts first an acidic α-proton and the resulting carbanion then displaces the halogen.
- The same sequence is observed in the Bingel reaction with fullerenes
- In crossed aldol reactions between haloketones and aldehydes the initial reaction product is a halohydrin which can subsequently form an oxirane in the presence of base.
- Haloketones are important in heterocyclic chemistry. An example is the use of haloketones in the Hantzsch pyrrole synthesis and the Hantzsch thiazole synthesis.
- Haloketones react with phosphites in the Perkow reaction.
- The halogroup can be removed in reductive dehalogenation of halo ketones
- Historically, treatment of haloketones with zinc dust in the Reformatsky reaction was one of the first reliable methods for generating unstabilized enolates. This has largely been superseded by bases such as lithium diisopropylamide.
See also
References
- The Chemistry of -Haloketones and Their Utility in Heterocyclic Synthesis Ayman W. Erian, Sherif M. Sherif and Hatem M. Gaber Molecules 2003, 8, 793-865 Online Article
- Scalable Methodology for the Catalytic, Asymmetric -Bromination of Acid Chlorides Cajetan Dogo-Isonagie, Tefsit Bekele, Stefan France, Jamison Wolfer, Anthony Weatherwax, Andrew E. Taggi, and Thomas Lectka J. Org. Chem.; 2006; 71(23) pp 8946 - 8949; (Note) doi:10.1021/jo061522l