Gypsy moths in the United States
The gypsy moth (Lymantria dispar) was introduced in 1868 into the United States by Étienne Léopold Trouvelot, a French scientist living in Medford, Massachusetts. Because native silk-spinning caterpillars were susceptible to disease, Trouvelot imported the species in order to breed a more resistant hybrid species. Some of the moths escaped, found suitable habitat, and began breeding. The gypsy moth is now a major pest of hardwood trees in the eastern United States.[1]
_(14598329900).jpg)

The first US outbreak occurred in 1889 around the eastern coast. In 1923 attempts were made to prevent the westward spread of the moth by maintaining a barrier zone extending from Canada to Long Island of nearly 27,300 km2. This barrier however broke down by 1939.[2] By 1987, the gypsy moth had established itself throughout the northeast US, southern Quebec, and Ontario. The insect has now spread into Michigan, Minnesota, Virginia, West Virginia, Illinois, and Wisconsin. Small, isolated infestations have sporadically occurred in Utah, Oregon, Washington, California, and British Columbia, but efforts have been taken to eradicate them.
Since 1980, the gypsy moth has defoliated over one million acres (4,000 km2) of forest each year. In 1981, 12.9 million acres (52,200 km2) were defoliated. In wooded suburban areas, during periods of infestation, gypsy moth larvae crawl over man-made obstacles and sometimes enter homes.[3] When feeding, they leave behind a mixture of small pieces of leaves and frass, or excrement. During outbreaks, the sound of moths chewing and dropping frass may be loud enough to sound like light to moderate rainfall.[4] Gypsy moth populations usually remain low, but occasional increases to very high levels can result in partial or total defoliation of host trees.[5]
According to a 2011 report, the gypsy moth is now one of the most destructive insects in the eastern United States; it and other foliage-eating pests cause an estimated $868 million in annual damages in the U.S.[6]
Host species
Gypsy moth larvae prefer oak trees, but may feed on many species of trees and shrubs, both hardwood and conifer. In the eastern US, the gypsy moth prefers oaks, aspen, apple, sweetgum, speckled alder, basswood, gray, paper birch, poplar, willow, and hawthorns, amongst other species. The gypsy moth avoids ash trees, tulip-tree, cucumber tree, American sycamore, butternut, black walnut, catalpa, flowering dogwood, balsam fir, cedar, American holly, and mountain laurel and rhododendron shrubs, but will feed on these in late instars when densities are extremely high. Older larvae feed on several species of softwood that younger larvae avoid, including cottonwood, hemlock, Atlantic white cypress, and pine and spruce species native to the east.[7]
Gypsy moth rash
The gypsy moth caterpillar has been reported to produce a poison ivy like rash when some people come into contact with the hairs of the larvae (caterpillar) stage. The contact can be direct or even indirect, if the small hairs are carried by the wind and onto the skin or clothing of a person. Gypsy moth rashes were documented in the early 1980s, during a major infestation in the Northeastern United States.[8] In coastal Maine and Cape Cod, Massachusetts, caterpillar-triggered rash is much more likely due to exposure to the browntail moth (Euproctis chrysorrhoea).[9]
Effects of defoliation

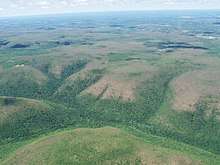
The effects of defoliation depend on the species of tree, amount of foliage removed, the health of the tree, the number of consecutive defoliations, and available soil moisture. If less than half of the crown is defoliated, most hardwood species will experience only a slight reduction in radial growth. If more than half of the crown is defoliated, most hardwoods will produce a second flush of foliage by midsummer. Healthy trees can usually withstand one or two consecutive major defoliations. Trees weakened by previous defoliation or subjected to other stresses like droughts are frequently killed after a single half-defoliation.
Trees use their energy reserves during re-foliation and may become weakened and exhibit symptoms such as the death of twigs and branches in the upper crown and sprouting of old buds on the trunk and larger branches. Weakened trees experience radial growth reduction of approximately 30 to 50 percent. Weakened trees are vulnerable to attack by disease organisms and other insects, or example, the Armillaria fungus may attack the roots, and the two-lined chestnut borer may attack the trunk and branches. Affected trees will eventually die two or three years after they are attacked by these pests.
Although not preferred by the larvae, pines and hemlocks are subject to heavy defoliation during gypsy moth outbreaks and are more likely to be killed than hardwoods. A complete defoliation can kill approximately half of pine species and 90 percent of mature hemlocks because conifers do not store energy in their roots; an exception is larch.
Factors that affect gypsy moth populations
Natural predators play an important role during periods of low population. Predators include wasps, flies, ground beetles, ants, many species of spider, several species of birds such as chickadees, blue jays, nuthatches, towhees, and robins and approximately 15 species of common woodland mammals, such as the white-footed mouse, shrews, chipmunks, squirrels, and raccoons. Small mammals are the largest predators in low density gypsy moth populations and are apparently critical in preventing outbreaks. Calosoma (ground beetles of European origin), cuckoos, and flocking birds such as starling, grackles, and red-winged blackbirds, are attracted to infested areas in high gypsy moth population years.[7]
Biological control
Diseases caused by bacteria, fungi, or viruses contribute to the decline of gypsy moth populations, especially during periods when populations are dense and are stressed by a lack of preferred foliage. Wilt disease caused by a particular nucleopolyhedrovirus (LdNPV) that is specific to the gypsy moth is its most devastating natural disease, causing a dramatic collapse of outbreak populations by killing both the larvae and pupae. Larvae infected with wilt disease are shiny and hang limply in an inverted "V" position. Infection with NPV is the most common source of mortality in high density populations and NPV epizootics usually cause the collapse of populations.[7]
Since the 1980s, the fungus Entomophaga maimaiga has also had a large impact on gypsy moth populations in North America.
Weather conditions can affect the survival and development of gypsy moth life stages, regardless of population density. Temperatures of −20 °F (−29 °C.) lasting from 48 to 72 hours can kill exposed eggs; alternate periods of freezing and thaw in late winter and early spring may prevent eggs from hatching; and cold, rainy weather inhibits dispersal and feeding and slows the growth of newly hatched larvae.[7]
Gypsy moth larvae have several predators which can help decrease their population. Lack of predation is one reason they can change from being a normal part of the ecosystem to an actual threat to trees. Among their predators are:
- Deer mice – are considered the most important predator of low-density gypsy moth populations and their abundance may be critical in determining whether populations go into an outbreak mode. Their abundance is strongly affected by the amount of mast (e.g., acorns) in the previous year.
- Tachinid flies – parasitize gypsy moth populations. While they may become quite abundant during a gypsy moth outbreak, they apparently have little effect on the population dynamics.
- Braconid wasps – also parasitize gypsy moths but play a minor role in their dynamics.[7]
The effectiveness of releasing or enhancing gypsy moth predators or parasites to control the moth is "difficult to determine", as rates of parasitism vary with the density of gypsy moth larvae, the range of alternate hosts for the parasite used, and the weather. Both in America and in Europe, research continues into biological control of the species, and for example the Baculoviridae viruses show potential for control.[10][11]
Management
Several methods of managing the gypsy moth are used; these include the monitoring of populations, maintaining the health and vigor of trees, concentrating and killing caterpillars, removing egg masses and treating with insecticides to kill larvae and protecting tree foliage. To concentrate the caterpillars, a strip of dark cloth about 12 inches wide – for example, burlap or old blue jeans, is tied around the tree at eye height. A string is tied around the cloth at its midpoint to create a fold of cloth around the tree. At mid-morning or later, the bands of cloth are checked for caterpillars, which are killed.
The egg masses – 3⁄4-inch long ovals that look like tan felt or velour – will be present from late July until May when they hatch. The egg masses are scraped off the tree and burnt or dumped in the trash. If they remain on the ground, the eggs will still be viable. Tactics suggested for homeowners may be too costly and labor-intensive for managers to use in forest stands.
The gypsy moth currently occupies less than a third of its potential range in North America and considerable resources are directed at minimizing its expansion into these areas. Every year, over 100,000 pheromone traps are placed in uninfested areas in order to detect new infestations that occasionally arise when people inadvertently transport life stages into uninfested areas (for example, egg masses on recreational vehicles). When captures are positive for several consecutive years, this indicates that a population is establishing and these are eradicated, usually via the application of the bacterial pesticide Bacillus thuringiensis ('Bt').[7]
In 2008 California agriculture officials quarantined a rural 5-square-mile (13 km2) section of Ventura County near Ojai to prevent the spread of a newly found gypsy moth colony.[12]
Pesticides
The decision to use pesticides is influenced by a number of factors, such as the quantity of visible egg masses, The percentage of preferred hosts, presence of dead or dying branches, and proximity to heavily infested woodlands. When numbers of gypsy moth larvae are high, pesticides may be the most effective method of killing larvae.
Available pesticides fall into two groups: microbial or biological and chemical (table 1). Microbial and biological pesticides contain living organisms that must be consumed by the pest. These include bacteria, viruses, and other organisms; biologicals include man-made synthetics of naturally occurring organisms. These pesticides are applied before the larvae reach the third stage of development. As they mature, larvae develop resistance to microbial pesticides. Low dose pheromone systems are used in Jersey, Channel Islands, to flood areas with synthetic pheromone and effectively 'blind' males so they are unable to locate females.[7]
Nucleopolyhedrovirus (NPV), a naturally occurring organism, has been developed as a microbial pesticide. It is presently registered under the name Gypchek and is available for use in USDA Forest Service sponsored suppression programs. NPV and Gypcheck are specific to the gypsy moth.
Bacillus thuringiensis (Bt) is microbial and biological. It is the most commonly used pesticide and is used against other pests, including the western spruce budworm and other Choristoneura and the tent caterpillar. When Bt is taken internally, the insect is paralyzed, stops feeding, and dies of starvation or disease.
Chemical pesticides are contact poisons and stomach poisons. The timing of application is less critical than that of microbials and biologicals. Chemical pesticides can affect non-target organisms and may be hazardous to human health.[7]
| Active ingredient | Representative trade names | Comments |
|---|---|---|
| Bacillus thuringiensis | Foray | Registered for aerial and ground application. Available under various trade names. Toxic to other moth and butterfly larvae. Can be used safely near water. |
| Acephate | Orthene | Registered for aerial and ground application. Available under various trade names. Toxic to bees and some gypsy moth parasites. Often used from the ground to treat individual trees. |
| Carbaryl | Sevin | Registered for aerial and ground application. Available under various trade names. Toxic to bees and gypsy moth parasites. Once the most widely used chemical in control programs. |
| Diflubenzuron | Dimilin | A restricted-use pesticide that can be applied only by certified applicators. |
The most commonly used chemical pesticides currently registered by the US Environmental Protection Agency (EPA) for use against the gypsy moth contain carbaryl, diflubenzuron, or acephate. Malathion, methoxychlor, phosmet, trichlorfon, and synthetic pyrethroids (permethrin) are registered by EPA but are used infrequently.
Several studies by Peter G. Kevan et al of the University of Guelph conducted between 1975 and 1995 showed serious reduction in pollination of blueberry and other crops due to aerial applications of insecticides that killed non-target wild bees. Diflubenzuron is an insect growth regulator and interferes with the normal molting process of the larvae but does not affect adult insects. Aquatic crustaceans and immature, non-target insects that undergo stages of molting are often sensitive to this pesticide.[7]
Mating disruption
Mating disruption of the gypsy moth, when used appropriately, effectively manages insect pest at different infestation levels. It can also be used alone or as a complement to other management methods like the use of conventional pesticides. Specifically, management of pests using mating disruption involves the use of synthetically created, chemically identical semiochemicals, in this case the species sex pheromone, that disrupt the mating behavior of the pest (most of the targeted pests belong to Lepidoptera and Coleoptera). Gypsy moth, Lymantria dispar, virgin females emit sex pheromones that attracts male gypsy moth, and mating ensues. By applying a large number of point sources that continuously emit naturally identical gypsy moth sex pheromone, or disparlure, male moths end up spending most of their time 'manipulating' these point sources, and thus have a significantly difficult time locating female moths, wasting time and effort following 'false' pheromone trails. This effectively reduces the number of mating encounters between adult moths.
Mating disruption has been successfully and safely used to manage gypsy moth in a number of eastern US states stretching from Wisconsin to North Carolina under the federal Slow the Spread (STS) program. Because mating disruption uses an insect's nature identical sex pheromone to disrupt their mating, mating disruption products are species-specific and do not impact other organisms and beneficial insects such as natural predators and pollinators. Hence they can be used as an alternative or complement to other control solutions, such as for the purpose of minimizing the negative effects of wide spectrum insecticides on non-target species and insecticide resistance build-up. Only Two mating disruption products have been approved by the USDA Forest Services for use in the STS program: Hercon Disrupt and ISCA Technologies's SPLAT GM.[13] These mating disruption products are formulated with the gypsy moth sex pheromone, and have been aerially applied to millions of acres since the early 1990s, which has successfully slowed the spread of the gypsy moth from the northeastern United States to the rest of the continent. SPLAT GM is also packaged and sold in handheld dispensers for manual application to smaller areas of 2.5 acres or more, and can be used as an alternative or complement to other gypsy moth control solutions.[14]
See also
Notes
- "Gypsy Moth". Wisconsin Department of Agriculture, Trade and Consumer Protection. Archived from the original on 31 August 2011. Retrieved 5 September 2011.
- McFadden, Max W.; McManus, Michael E. (1991). "An insect out of control? The potential for spread and establishment of the Gypsy moth in new forest areas in the United States". In Baranchikov, Y.N.; Mattson, W.J.; Hain, F.P.; Payne, T.L (eds.). Forest Insect Guilds: Patterns of Interaction with Host Trees (PDF). U.S. Dep. Agric. For. Serv. Gen. Tech. Rep. NE-153.
- M. McManus; N. Schneeberger; R. Reardon; G. Mason (October 1989). "Forest Insect & Disease Leaflet 162 – Gypsy Moth". U.S. Department of Agriculture Forest Service. Retrieved 2010-07-10.
- "How and Why Gypsy Moth Treatment Sites are Selected" (PDF). Wisconsin Cooperative Gypsy Moth Program. Retrieved 2010-07-10.
- "An Atlas of Historical Gypsy Moth Defoliation & Quarantined Areas in the US". USDA Forest Service. 2003-02-05. Retrieved 2010-07-10.
- Aukema, Juliann E.; Leung, Brian; Kovacs, Kent; Chivers, Corey; Britton, Kerry O.; Englin, Jeffrey; Frankel, Susan J.; Haight, Robert G.; Holmes, Thomas P.; Liebhold, Andrew M.; McCullough, Deborah G.; Von Holle, Betsy; Gratwicke, Brian (September 9, 2011). Gratwicke, Brian (ed.). "Economic Impacts of Non-Native Forest Insects in the Continental United States". PLoS ONE. 6 (9): e24587. Bibcode:2011PLoSO...624587A. doi:10.1371/journal.pone.0024587. PMC 3170362. PMID 21931766. Retrieved September 8, 2012. Lay summary – Journalist's Resource (September 15, 2011).
- M. McManus; N. Schneeberger; R. Reardon; G. Mason (August 1992). "Forest Insect& Disease Leaflet 162: Gypsy Moth". U.S. Department of Agriculture Forest Service. Retrieved 5 September 2011.
- Tuthill, R W; Canada, A T; Wilcock, K; Etkind, P H; O'Dell, T M (1984-08-01). "An epidemiologic study of gypsy moth rash". American Journal of Public Health. 74: 799–803. doi:10.2105/ajph.74.8.799. PMC 1651967. PMID 6742270.
- Browntail moth Maine Forest Service (2018).
- Reardon, Richard C. "Biological Control of The Gypsy Moth: An Overview". Southern Appalachian Biological Control Initiative Workshop. Retrieved 10 April 2017.
- "Gypsy moth". Biocomes. Retrieved 10 April 2017.
- "Gypsy moth leads to Ventura County area quarantine". SignOnSanDiego.com. Associated Press. 2008-10-30. Retrieved 2010-07-10.
- "Effects of SPLAT GM Sprayable Pheromone on Gypsy Moth Mating Success", USDA Forest Service - National Agroforestry Center. Retrieved 17 January 2011.
- "Archived copy". Archived from the original on 2012-05-08. Retrieved 2012-04-12.CS1 maint: archived copy as title (link)
References
![]()
Further reading
- Andreadis, T.G.; Weseloh, R.M. (1990). "Discovery of Entomophaga maimaiga in North American gypsy moth, Lymantria dispar". PNAS. 87 (7): 2461–2465. Bibcode:1990PNAS...87.2461A. doi:10.1073/pnas.87.7.2461. PMC 53709.
- Bogdanowicz, S.M.; Wallner, W.E.; Bell, J.; et al. (1993). "Asian gypsy moths (Lepidoptera, Lymantriidae) in North America — evidence from molecular data". Annals of the Entomological Society of America. 86 (6): 710–715.
- McManus, M.; Schneeberger, N.; Reardon, R. & Mason, G. (1992): Gypsy Moth. Forest Insect & Disease Leaflet 162 U.S. Department of Agriculture Forest Service.
- Liebhold, A.M.; Halverson, J.A.; Elmes, G.A. (1992). "Gypsy moth invasion in North America — a quantitative analysis". Journal of Biogeography. 19 (5): 513–520. doi:10.2307/2845770. JSTOR 2845770.
- McManus, Michael L.; Houston, David R. & Wallner, William E. (1979): The homeowner and the gypsy moth: Guidelines for control. Home and Gard. Bull. 227: 4–33. U.S. Department of Agriculture, Washington DC.
- Myers, Judith H. (1993). "Population Outbreaks in Forest Lepidoptera". American Scientist. 81: 240–251. Bibcode:1993AmSci..81..240M.
- Podgwaite, J.D. (1979): Diseases of the gypsy moth: How they help to regulate populations. Agric. Handb. 539: 2–15. U.S. Department of Agriculture, Washington DC.
- United States Department of Agriculture, Forest Service (1985). Gypsy Moth Suppression and Eradication Projects (as Supplemented 1985): Environmental Impact Statement.
External links
| Wikimedia Commons has media related to Lymantria dispar. |
- asiangypsymoth.org
- Gypsy moth in North America at the USDA Forest Service
- Gypsy Moth Slow the Spread Foundation, Inc.
- "Gypsy moth leads to Ventura County area quarantine". legacy.utsandiego.com. The San Diego Union-Tribune. Associated Press. October 30, 2008. Retrieved September 8, 2012.