Electronvolt
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the amount of kinetic energy gained by a single electron accelerating from rest through an electric potential difference of one volt in vacuum. When used as a unit of energy, the numerical value of 1 eV in joules (symbol J) is equivalent to the numerical value of the charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value 1.602176634×10−19 J.[1]
Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge q has an energy E = qV after passing through the potential V; if q is quoted in integer units of the elementary charge and the potential in volts, one gets an energy in eV.
It is a common unit of energy within physics, widely used in solid state, atomic, nuclear, and particle physics. It is commonly used with the metric prefixes milli-, kilo-, mega-, giga-, tera-, peta- or exa- (meV, keV, MeV, GeV, TeV, PeV and EeV respectively). In some older documents, and in the name Bevatron, the symbol BeV is used, which stands for billion (109) electronvolts; it is equivalent to the GeV.
| Measurement | Unit | SI value of unit |
|---|---|---|
| Energy | eV | 1.602176634×10−19 J |
| Mass | eV/c2 | 1.782662×10−36 kg |
| Momentum | eV/c | 5.344286×10−28 kg·m/s |
| Temperature | eV/kB | 1.160451812×104 K |
| Time | ħ/eV | 6.582119×10−16 s |
| Distance | ħc/eV | 1.97327×10−7 m |
Definition
An electronvolt is the amount of kinetic energy gained or lost by a single electron accelerating from rest through an electric potential difference of one volt in vacuum. Hence, it has a value of one volt, 1 J/C, multiplied by the electron's elementary charge e, 1.602176634×10−19 C.[2] Therefore, one electronvolt is equal to 1.602176634×10−19 J.[3]
The electronvolt, as opposed to the volt, is not an SI unit. The electronvolt (eV) is a unit of energy whereas the volt (V) is the derived SI unit of electric potential. The SI unit for energy is the joule (J).
Mass
By mass–energy equivalence, the electronvolt is also a unit of mass. It is common in particle physics, where units of mass and energy are often interchanged, to express mass in units of eV/c2, where c is the speed of light in vacuum (from E = mc2). It is common to simply express mass in terms of "eV" as a unit of mass, effectively using a system of natural units with c set to 1.[4] The mass equivalent of 1 eV/c2 is
For example, an electron and a positron, each with a mass of 0.511 MeV/c2, can annihilate to yield 1.022 MeV of energy. The proton has a mass of 0.938 GeV/c2. In general, the masses of all hadrons are of the order of 1 GeV/c2, which makes the GeV (gigaelectronvolt) a convenient unit of mass for particle physics:
- 1 GeV/c2 = 1.78266192×10−27 kg.
The unified atomic mass unit (u), almost exactly 1 gram divided by the Avogadro number, is almost the mass of a hydrogen atom, which is mostly the mass of the proton. To convert to electron volts, use the formula:
- 1 u = 931.4941 MeV/c2 = 0.9314941 GeV/c2.
Momentum
In high-energy physics, the electronvolt is often used as a unit of momentum. A potential difference of 1 volt causes an electron to gain an amount of energy (i.e., 1 eV). This gives rise to usage of eV (and keV, MeV, GeV or TeV) as units of momentum, for the energy supplied results in acceleration of the particle.
The dimensions of momentum units are LMT−1. The dimensions of energy units are L2MT−2. Then, dividing the units of energy (such as eV) by a fundamental constant that has units of velocity (LT−1), facilitates the required conversion of using energy units to describe momentum. In the field of high-energy particle physics, the fundamental velocity unit is the speed of light in vacuum c.
By dividing energy in eV by the speed of light, one can describe the momentum of an electron in units of eV/c.[5] [6]
The fundamental velocity constant c is often dropped from the units of momentum by way of defining units of length such that the value of c is unity. For example, if the momentum p of an electron is said to be 1 GeV, then the conversion to MKS can be achieved by:
Distance
In particle physics, a system of "natural units" in which the speed of light in vacuum c and the reduced Planck constant ħ are dimensionless and equal to unity is widely used: c = ħ = 1. In these units, both distances and times are expressed in inverse energy units (while energy and mass are expressed in the same units, see mass–energy equivalence). In particular, particle scattering lengths are often presented in units of inverse particle masses.
Outside this system of units, the conversion factors between electronvolt, second, and nanometer are the following:
The above relations also allow expressing the mean lifetime τ of an unstable particle (in seconds) in terms of its decay width Γ (in eV) via Γ = ħ/τ. For example, the B0 meson has a lifetime of 1.530(9) picoseconds, mean decay length is cτ = 459.7 μm, or a decay width of (4.302±25)×10−4 eV.
Conversely, the tiny meson mass differences responsible for meson oscillations are often expressed in the more convenient inverse picoseconds.
Energy in electronvolts is sometimes expressed through the wavelength of light with photons of the same energy:
Temperature
In certain fields, such as plasma physics, it is convenient to use the electronvolt to express temperature. The electronvolt is divided by the Boltzmann constant to convert to the Kelvin scale:
Where kB is the Boltzmann constant, K is Kelvin, J is Joules, eV is electronvolts.
The kB is assumed when using the electronvolt to express temperature, for example, a typical magnetic confinement fusion plasma is 15 keV (kilo-electronvolts), which is equal to 170 MK (million Kelvin).
As an approximation: kBT is about 0.025 eV (≈ 290 K/11604 K/eV) at a temperature of 20 °C.
Properties
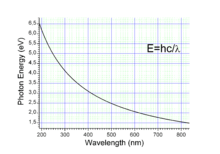
The energy E, frequency v, and wavelength λ of a photon are related by
where h is the Planck constant, c is the speed of light. This reduces to
A photon with a wavelength of 532 nm (green light) would have an energy of approximately 2.33 eV. Similarly, 1 eV would correspond to an infrared photon of wavelength 1240 nm or frequency 241.8 THz.
Scattering experiments
In a low-energy nuclear scattering experiment, it is conventional to refer to the nuclear recoil energy in units of eVr, keVr, etc. This distinguishes the nuclear recoil energy from the "electron equivalent" recoil energy (eVee, keVee, etc.) measured by scintillation light. For example, the yield of a phototube is measured in phe/keVee (photoelectrons per keV electron-equivalent energy). The relationship between eV, eVr, and eVee depends on the medium the scattering takes place in, and must be established empirically for each material.
Energy comparisons
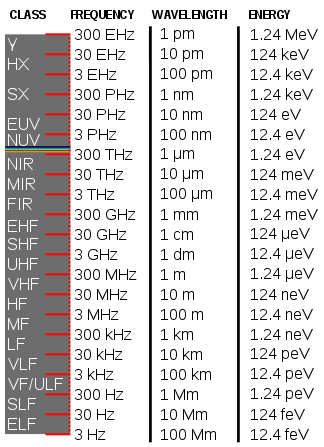
| γ: Gamma rays | MIR: Mid infrared | HF: High freq. |
| HX: Hard X-rays | FIR: Far infrared | MF: Medium freq. |
| SX: Soft X-rays | Radio waves | LF: Low freq. |
| EUV: Extreme ultraviolet | EHF: Extremely high freq. | VLF: Very low freq. |
| NUV: Near ultraviolet | SHF: Super high freq. | VF/ULF: Voice freq. |
| Visible light | UHF: Ultra high freq. | SLF: Super low freq. |
| NIR: Near Infrared | VHF: Very high freq. | ELF: Extremely low freq. |
| Freq: Frequency |
| Energy | Source |
|---|---|
| 5.25×1032 eV | total energy released from a 20 kt nuclear fission device |
| 1.22×1028 eV | the Planck energy |
| 10 YeV (1×1025 eV) | approximate grand unification energy |
| ~624 EeV (6.24×1020 eV) | energy consumed by a single 100-watt light bulb in one second (100 W = 100 J/s ≈ 6.24×1020 eV/s) |
| 300 EeV (3×1020 eV = ~50 J) | [10] the so-called Oh-My-God particle (the most energetic cosmic ray particle ever observed) |
| 2 PeV | two petaelectronvolts, the most high-energetic neutrino detected by the IceCube neutrino telescope in Antarctica[11] |
| 14 TeV | designed proton collision energy at the Large Hadron Collider (operated at about half of this energy since 30 March 2010, reached 13 TeV in May 2015) |
| 1 TeV | a trillion electronvolts, or 1.602×10−7 J, about the kinetic energy of a flying mosquito[12] |
| 172 GeV | rest energy of top quark, the heaviest measured elementary particle |
| 125.1±0.2 GeV | energy corresponding to the mass of the Higgs boson, as measured by two separate detectors at the LHC to a certainty better than 5 sigma[13] |
| 210 MeV | average energy released in fission of one Pu-239 atom |
| 200 MeV | approximate average energy released in nuclear fission fission fragments of one U-235 atom. |
| 105.7 MeV | rest energy of a muon |
| 17.6 MeV | average energy released in the fusion of deuterium and tritium to form He-4; this is 0.41 PJ per kilogram of product produced |
| 2 MeV | approximate average energy released in a nuclear fission neutron released from one U-235 atom. |
| 1.9 MeV | rest energy of up quark, the lowest mass quark. |
| 1 MeV (1.602×10−13 J) | about twice the rest energy of an electron |
| 1 to 10 keV | approximate thermal temperature, , in nuclear fusion systems, like the core of the sun, magnetically confined plasma, inertial confinement and nuclear weapons |
| 13.6 eV | the energy required to ionize atomic hydrogen; molecular bond energies are on the order of 1 eV to 10 eV per bond |
| 1.6 eV to 3.4 eV | the photon energy of visible light |
| < 2 eV | approximate rest energy of neutrinos |
| 1.1 eV | energy required to break a covalent bond in silicon |
| 720 meV | energy required to break a covalent bond in germanium |
| 25 meV | thermal energy, , at room temperature; one air molecule has an average kinetic energy 38 meV |
| 230 μeV | thermal energy, , of the cosmic microwave background |
Per mole
One mole of particles given 1 eV of energy has approximately 96.5 kJ of energy — this corresponds to the Faraday constant (F ≈ 96485 C mol−1), where the energy in joules of n moles of particles each with energy E eV is equal to E·F·n.
See also
References
- "CODATA Value: Planck constant in eV s". Archived from the original on 22 January 2015. Retrieved 30 March 2015.
- "2018 CODATA Value: elementary charge". The NIST Reference on Constants, Units, and Uncertainty. NIST. 20 May 2019. Retrieved 2019-05-20.
- "2018 CODATA Value: electron volt". The NIST Reference on Constants, Units, and Uncertainty. NIST. 20 May 2019. Retrieved 2019-05-20.
- Barrow, J. D. "Natural Units Before Planck." Quarterly Journal of the Royal Astronomical Society 24 (1983): 24.
- "Units in particle physics". Associate Teacher Institute Toolkit. Fermilab. 22 March 2002. Archived from the original on 14 May 2011. Retrieved 13 February 2011.
- "Special Relativity". Virtual Visitor Center. SLAC. 15 June 2009. Retrieved 13 February 2011.
- What is Light? Archived December 5, 2013, at the Wayback Machine – UC Davis lecture slides
- Elert, Glenn. "Electromagnetic Spectrum, The Physics Hypertextbook". hypertextbook.com. Archived from the original on 2016-07-29. Retrieved 2016-07-30.
- "Definition of frequency bands on". Vlf.it. Archived from the original on 2010-04-30. Retrieved 2010-10-16.
- Open Questions in Physics. Archived 2014-08-08 at the Wayback Machine German Electron-Synchrotron. A Research Centre of the Helmholtz Association. Updated March 2006 by JCB. Original by John Baez.
- "A growing astrophysical neutrino signal in IceCube now features a 2-PeV neutrino". Archived from the original on 2015-03-19.
- Glossary Archived 2014-09-15 at the Wayback Machine - CMS Collaboration, CERN
- ATLAS; CMS (26 March 2015). "Combined Measurement of the Higgs Boson Mass in pp Collisions at √s=7 and 8 TeV with the ATLAS and CMS Experiments". Physical Review Letters. 114 (19): 191803. arXiv:1503.07589. Bibcode:2015PhRvL.114s1803A. doi:10.1103/PhysRevLett.114.191803. PMID 26024162.
