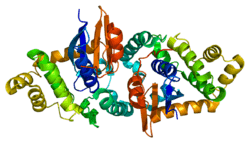GSTK1
Glutathione S-transferase kappa 1 (GSTK1) is an enzyme that in humans is encoded by the GSTK1 gene which is located on chromosome seven.[5] It belongs to the superfamily of enzymes known as glutathione S-transferase (GST), which are mainly known for cellular detoxification.[6] The GSTK1 gene consists of eight exons and seven introns and although it is a member of the GST family, its structure has been found to be similar to bacterial HCCA (2-hydroxychromene-2-carboxylate) isomerases and bacterial disulphide-bond-forming DsbA oxidoreductase. This similarity has later allowed the enzyme GSTK1 to be renamed to DsbA-L.[7] Research has also suggested that several variations of the GSTK1 gene can be responsible for metabolic diseases and certain types of cancer.[7]
Structure
The GSTK1 enzyme is a homodimer and, like all GSTs, it contains a TRX-like domain and a helical domain. However, the GSTK1 is substantially different in its secondary structure compared to the other GSTs. The helical domain has been observed to be placed between the βαβ and ββα motifs of the TRX-like domain, rather than the TRX-like domain and the C-terminal helical domain being connected together by a short linker of alpha-helixes as normally seen in GSTs.[6] Also, the GSTK1 dimer employs a butterfly shape and not a V-shaped crevice like in the other classes.[6] As for the GSTK1 gene, it is ~5 kb long, has eight exons, is located on chromosome 7q34, and includes an initiator element at the transcription start site instead of a TATA or a CCAAT box.[5]
Function
GSTK1 promotes adiponectin multimerization in the endoplasmic reticulum (ER) though the mechanism by which this occurs is unknown.[8] GSTK1 can prevent ER stress and ER stress-induced adiponectin down-regulation, implying that GSTK1 assists the ER’s functions. GSTK1 is located in the ER and also in the mitochondria of hepatocytes. This indicates a potential role for GSTK1 in the interaction between the two organelles; though this is poorly understood.[8]
The discovery of GSTK1 in the peroxisome has led to studies based on its function. It has been suggested that, similar to GSTA, GSTK1 may play a role in the buffering of acyl-CoA and xenobiotic-CoA and be involved in their binding activities. GSTK1 may also be responsible for the detoxification of lipid peroxides created in the peroxisome based on the peroxidase activity towards three substrates: tert-butyl hydroperoxide, cumene hydroperoxide, and 15-S-hydroperoxy-5,8,11,13-eicosatetraenoic acid.[5]
Clinical significance
The amount of expression of adiponectin has been observed to be related to diseases such as insulin resistance, obesity, and type 2 diabetes. Decreased amounts of the protein indicates that there is a higher probability of receiving said diseases. Because the GSTK1 is seen to play a role in the multimerization of adiponectin, this enzyme can regulate the concentration of adiponectin and thus enhance insulin sensitivity and protect against diabetes.[7] Also, the GSTK1 gene is unregulated when it is inflicted with oxidative stress and are over expressed in many tumors leading to difficulties during cancer chemotherapy.[9] Moreover, GSTK1 gene expression has been seen to increase significantly in correlation to drug resistance in tumor cells such as erythroleukemia and mammary adenocarcinoma suggesting that it, along with GSTP1 and GSTA4, could be responsible for the drug resistance.[10]
GSTK1 can also be a potential tool to help investigate cancer. Tyrosine phosphorylated proteins are responsible for many of the cell functions such as the cell’s growth, division, adhesion, and motility. These activities are also very related to cancer and thus studying this protein could allow access to information which could classify tumors for prognosis and prediction.[11] Due to GSTK1’s C-terminal SH2 domain, tyrosine phosphorylated proteins can bind to it and allow for easier detection to which the protein can be studied.[11]
Interactions
GSTK1 has been seen to interact with:
References
- GRCh38: Ensembl release 89: ENSG00000197448 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000029864 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Morel F, Rauch C, Petit E, Piton A, Theret N, Coles B, Guillouzo A (Apr 2004). "Gene and protein characterization of the human glutathione S-transferase kappa and evidence for a peroxisomal localization". The Journal of Biological Chemistry. 279 (16): 16246–53. doi:10.1074/jbc.M313357200. PMID 14742434.
- Li J, Xia Z, Ding J (Sep 2005). "Thioredoxin-like domain of human kappa class glutathione transferase reveals sequence homology and structure similarity to the theta class enzyme". Protein Science. 14 (9): 2361–9. doi:10.1110/ps.051463905. PMC 2253485. PMID 16081649.
- Gao F, Fang Q, Zhang R, Lu J, Lu H, Wang C, Ma X, Xu J, Jia W, Xiang K (2009). "Polymorphism of DsbA-L gene associates with insulin secretion and body fat distribution in Chinese population". Endocrine Journal. 56 (3): 487–94. doi:10.1507/endocrj.k08e-322. PMID 19225211.
- Liu M, Chen H, Wei L, Hu D, Dong K, Jia W, Dong LQ, Liu F (Apr 2015). "Endoplasmic reticulum (ER) localization is critical for DsbA-L protein to suppress ER stress and adiponectin down-regulation in adipocytes". The Journal of Biological Chemistry. 290 (16): 10143–8. doi:10.1074/jbc.M115.645416. PMC 4400330. PMID 25739441.
- Nebert DW, Vasiliou V (Nov 2004). "Analysis of the glutathione S-transferase (GST) gene family". Human Genomics. 1 (6): 460–4. doi:10.1186/1479-7364-1-6-460. PMC 3500200. PMID 15607001.
- Kalinina EV, Berozov TT, Shtil AA, Chernov NN, Glasunova VA, Novichkova MD, Nurmuradov NK (Nov 2012). "Expression of genes of glutathione transferase isoforms GSTP1-1, GSTA4-4, and GSTK1-1 in tumor cells during the formation of drug resistance to cisplatin". Bulletin of Experimental Biology and Medicine. 154 (1): 64–7. doi:10.1007/s10517-012-1876-4. PMID 23330092.
- Qiu F, Huang D, Xiao H, Qiu F, Lu L, Nie J (Apr 2013). "Detection of tyrosine‑phosphorylated proteins in hepatocellular carcinoma tissues using a combination of GST‑Nck1‑SH2 pull‑down and two‑dimensional electrophoresis". Molecular Medicine Reports. 7 (4): 1209–14. doi:10.3892/mmr.2013.1324. PMID 23426619.
Further reading
- Zhang QH, Ye M, Wu XY, Ren SX, Zhao M, Zhao CJ, Fu G, Shen Y, Fan HY, Lu G, Zhong M, Xu XR, Han ZG, Zhang JW, Tao J, Huang QH, Zhou J, Hu GX, Gu J, Chen SJ, Chen Z (Oct 2000). "Cloning and functional analysis of cDNAs with open reading frames for 300 previously undefined genes expressed in CD34+ hematopoietic stem/progenitor cells". Genome Research. 10 (10): 1546–60. doi:10.1101/gr.140200. PMC 310934. PMID 11042152.
- Hartley JL, Temple GF, Brasch MA (Nov 2000). "DNA cloning using in vitro site-specific recombination". Genome Research. 10 (11): 1788–95. doi:10.1101/gr.143000. PMC 310948. PMID 11076863.
- Wiemann S, Weil B, Wellenreuther R, Gassenhuber J, Glassl S, Ansorge W, Böcher M, Blöcker H, Bauersachs S, Blum H, Lauber J, Düsterhöft A, Beyer A, Köhrer K, Strack N, Mewes HW, Ottenwälder B, Obermaier B, Tampe J, Heubner D, Wambutt R, Korn B, Klein M, Poustka A (Mar 2001). "Toward a catalog of human genes and proteins: sequencing and analysis of 500 novel complete protein coding human cDNAs". Genome Research. 11 (3): 422–35. doi:10.1101/gr.GR1547R. PMC 311072. PMID 11230166.
- Simpson JC, Wellenreuther R, Poustka A, Pepperkok R, Wiemann S (Sep 2000). "Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing". EMBO Reports. 1 (3): 287–92. doi:10.1093/embo-reports/kvd058. PMC 1083732. PMID 11256614.
- Robinson A, Huttley GA, Booth HS, Board PG (May 2004). "Modelling and bioinformatics studies of the human Kappa-class glutathione transferase predict a novel third glutathione transferase family with similarity to prokaryotic 2-hydroxychromene-2-carboxylate isomerases". The Biochemical Journal. 379 (Pt 3): 541–52. doi:10.1042/BJ20031656. PMC 1224102. PMID 14709161.
- Wiemann S, Arlt D, Huber W, Wellenreuther R, Schleeger S, Mehrle A, Bechtel S, Sauermann M, Korf U, Pepperkok R, Sültmann H, Poustka A (Oct 2004). "From ORFeome to biology: a functional genomics pipeline". Genome Research. 14 (10B): 2136–44. doi:10.1101/gr.2576704. PMC 528930. PMID 15489336.
- Li J, Xia Z, Ding J (Sep 2005). "Thioredoxin-like domain of human kappa class glutathione transferase reveals sequence homology and structure similarity to the theta class enzyme". Protein Science. 14 (9): 2361–9. doi:10.1110/ps.051463905. PMC 2253485. PMID 16081649.
- Mehrle A, Rosenfelder H, Schupp I, del Val C, Arlt D, Hahne F, Bechtel S, Simpson J, Hofmann O, Hide W, Glatting KH, Huber W, Pepperkok R, Poustka A, Wiemann S (Jan 2006). "The LIFEdb database in 2006". Nucleic Acids Research. 34 (Database issue): D415-8. doi:10.1093/nar/gkj139. PMC 1347501. PMID 16381901.
- Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.