Fritsch–Buttenberg–Wiechell rearrangement
The Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch (1859–1913), Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction whereby a 1,1-diaryl-2-bromo-alkene rearranges to a 1,2-diaryl-alkyne by reaction with a strong base such as an alkoxide.[1][2][3][4]
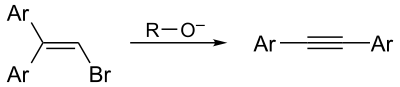
The Fritsch-Buttenberg-Wiechell rearrangement
| Fritsch–Buttenberg–Wiechell rearrangement | |
|---|---|
| Named after | Paul Ernst Moritz Fritsch Wilhelm Paul Buttenberg Heinrich G. Wiechell |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000250 |
This rearrangement is also possible with alkyl substituents.[5]
Reaction mechanism
The strong base deprotonates the vinylic hydrogen, which after alpha-elimination forms a vinyl carbene. A 1,2-aryl migration forms the 1,2-diaryl-alkyne product. The mechanism of the FBW rearrangement was a subject of on-surface studies where the vinyl radical was visualised with sub-atomic resolution.[6]

Mechanism of the Fritsch-Buttenberg-Wiechell rearrangement
Scope
One study explored this reaction for the synthesis of novel polyynes:[7][8]
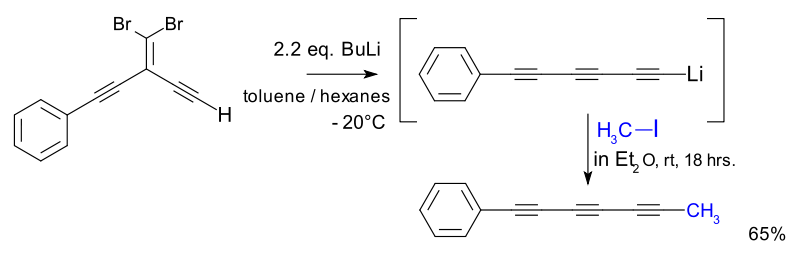
Fritsch-Buttenberg-Wiechell rearrangement Application
gollark: Gives me ultimate cosmic power over reality, why?
gollark: I'm sure there are at least 10 of those.
gollark: I mean, infohazards have been done a ton and there's at least two anomalies vaguely involving Discord.
gollark: Sounds like a generic boring infohazard.
gollark: What a valid* SCP number.
See also
References
- Paul Fritsch (1894). "Ueber die Darstellung von Diphenylacetaldehyd und eine neue Synthese von Tolanderivaten". Justus Liebig's Annalen der Chemie. 279 (3): 319–323. doi:10.1002/jlac.18942790310.
- Buttenberg, W. P. (1894). "Condensation des Dichloracetals mit Phenol und Toluol". Justus Liebig's Annalen der Chemie. 279 (3): 324–337. doi:10.1002/jlac.18942790311.
- Wiechell, H. (1894). "Condensation des Dichloracetals mit Anisol und Phenetol". Justus Liebig's Annalen der Chemie. 279 (3): 337–344. doi:10.1002/jlac.18942790312.
- Köbrich, G. (1965). "Eliminations from Olefins". Angewandte Chemie International Edition. 4: 49–68. doi:10.1002/anie.196500491.
- Rezaei, H.; Yamanoi, S.; Chemla, F.; Normant, J. F. (2000). "Fritsch-Buttenberg-Wiechell Rearrangement in the Aliphatic Series". Org. Lett. 2 (4): 419–421. doi:10.1021/ol991117z. PMID 10814340.
- Pavliček, Niko; Gawel, Przemyslaw; Kohn, Daniel R.; Majzik, Zsolt; Xiong, Yaoyao; Meyer, Gerhard; Anderson, Harry L.; Gross, Leo (2018-07-02). "Polyyne formation via skeletal rearrangement induced by atomic manipulation". Nature Chemistry. 10 (8): 853–858. doi:10.1038/s41557-018-0067-y. ISSN 1755-4330. PMC 6071858. PMID 29967394.
- One-Pot Formation and Derivatization of Di- and Triynes Based on the Fritsch-Buttenberg-Wiechell Rearrangement Thanh Luu, Yasuhiro Morisaki, Nina Cunningham, and Rik R. Tykwinski J. Org. Chem. 2007, 72, 9622–9629 doi:10.1021/jo701810g
- The metal acetylide intermediate is captured by electrophile methyl iodide. The reaction product is a biomolecule found in for instance Bidens pilosa
- Darses, B.; Milet, A.; Philouze, C.; Greene, A. E.; Poisson, J.-F. o., Ynol Ethers from Dichloroenol Ethers: Mechanistic Elucidation Through 35Cl Labeling. Organic Letters 2008, 10 (20), 4445-4447.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.