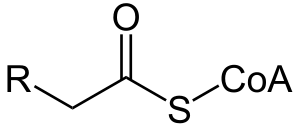
Acyl-CoA
Acyl-CoA is a group of coenzymes that metabolize fatty acids. Acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way, fats are converted to ATP, the universal biochemical energy carrier.
Functions
Fatty acid activation
The oxidative degradation of fatty acids is a two-step process, catalyzed by acyl-CoA synthetase. First, the fatty acid reacts with ATP to form an acyl phosphate. This intermediate reacts subsequently to give acyl-CoA:
- Fatty acid + CoA + ATP ⇌ Acyl-CoA + AMP + PPi
Fatty acids are activated in the cytosol, but oxidation occurs in the mitochondria. Because there is no transport protein for CoA adducts, acyl groups must enter the mitochondria via a shuttle system involving the small molecule carnitine.[1]
Acyl-CoA is made by an enzyme called Acyl-CoA synthase. There are three different types of acyl-CoA synthases[2] which can help make 3 different lengths of acyl-CoA. For example, medium chain acyl-CoA synthase is made to work upon 4-11 carbon fatty acids to make 4-11 carbon acyl-CoA. A different type of Acyl-CoA synthase is used for a 11-20 carbon fatty acid to make it into a 11-20 Acyl-CoA. The reaction to make Acyl-CoA is also thermodynamically favored because in this reaction ATP becomes AMP[3] which is a two step reaction, and this is spontaneous. There is also an enzyme called Acyl-CoA thioesterase, and this enzyme does the opposite of Acyl-CoA synthase. This enzyme takes of the Acyl-CoA to form a free fatty acid and coenzyme A, in other words deactivates the fatty acid by breaking down Acyl-CoA.[3]
Clinical Significance
Heart muscle primarily metabolizes fat for energy and Acyl-CoA metabolism has been identified[4] as a critical molecule in early stage heart muscle pump failure.
Cellular acyl-CoA content correlates with insulin resistance, suggesting that it can mediate lipotoxicity in non-adipose tissues.[5] Acyl-CoA: diacylglycerol acyltransferase (DGAT) plays an important role in energy metabolism on account of key enzyme in triglyceride biosynthesis. The synthetic role of DGAT in adipose tissue such as the liver and the intestine, sites where endogenous levels of its activity and triglyceride synthesis are high and comparatively clear. Also, any changes in the activity levels might cause changes in systemic insulin sensitivity and energy homeostasis.[6]
A rare disease called multiple acyl-CoA dehydrogenase deficiency (MADD)[7] is a fatty acid metabolism disorder. Acyl-CoA is important because this enzyme helps make Acyl-CoA from free fatty acids, and this activates the fatty acid to be metabolized. This compromised fatty acid oxidation leads to many different symptoms, including severe symptoms such as cardiomyopathy and liver disease and mild symptoms such as episodic metabolic decomposition, muscle weakness and respiratory failure. MADD is a genetic disorder, caused by a mutation in the ETFA, ETFB, and ETFDH genes. MADD is known as an “autosomal recessive disorder”[7] because for one to get this disorder, one must receive this recessive gene from both parents.
See also
- Acetyl-CoA
- Beta oxidation
- Coenzyme A
- Acyl CoA dehydrogenase
- Fatty acid metabolism
References
- Pratt C.W., Cornely, K. Essential Biochemistry. John Wiley & Sons, Inc. (2004)
- Blanco, Antonio; Blanco, Gustavo (2017). "Lipid Metabolism". Medical Biochemistry. pp. 325–365. doi:10.1016/B978-0-12-803550-4.00015-X. ISBN 978-0-12-803550-4.
- Bhagavan, N.V.; Ha, Chung-Eun (2015). "Lipids I: Fatty Acids and Eicosanoids". Essentials of Medical Biochemistry. pp. 269–297. doi:10.1016/B978-0-12-416687-5.00016-6. ISBN 978-0-12-416687-5.
- Goldenberg, Joseph R.; Carley, Andrew N.; Ji, Ruiping; Zhang, Xiaokan; Fasano, Matt; Schulze, P. Christian; Lewandowski, E. Douglas (26 March 2019). "Preservation of Acyl-CoA Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking". Circulation. 139 (24): 2765–2777. doi:10.1161/CIRCULATIONAHA.119.039610. PMC 6557671. PMID 30909726. Lay summary – Ohio State News (March 26, 2019).
- Li, Lei O.; Klett, Eric L.; Coleman, Rosalind A. (March 2010). "Acyl-CoA synthesis, lipid metabolism and lipotoxicity". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1801 (3): 246–251. doi:10.1016/j.bbalip.2009.09.024. PMC 2824076. PMID 19818872.
- Yu, Yi‐Hao; Ginsberg, Henry (8 July 2009). "The role of acyl‐CoA:diacylglycerol acyltransferase (DGAT) in energy metabolism". Annals of Medicine. 36 (4): 252–261. doi:10.1080/07853890410028429. PMID 15224651.
- Rashmi, S.; Gayathri, N.; Kumar, M. Veerendra; Sumanth, S.; Subasree, R.; Pooja, M. (1 January 2017). "Multiple Acyl CoA dehydrogenase deficiency: Uncommon yet treatable disorder". Neurology India. 65 (1): 177–8. doi:10.4103/0028-3886.198186 (inactive 2020-05-25). PMID 28084266.
External links
- Acyl+Coenzyme+A at the US National Library of Medicine Medical Subject Headings (MeSH)