Ferroelectricity
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field.[1][2] All ferroelectrics are pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Valasek.[3] Thus, the prefix ferro, meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric and ferromagnetic are known as multiferroics.
Polarization
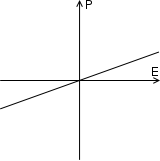
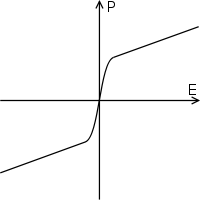
When most materials are polarized, the polarization induced, P, is almost exactly proportional to the applied external electric field E; so the polarization is a linear function. This is called linear dielectric polarization (see figure). Some materials, known as paraelectric materials,[4] show a more enhanced nonlinear polarization (see figure). The electric permittivity, corresponding to the slope of the polarization curve, is not constant as in linear dielectrics but is a function of the external electric field.
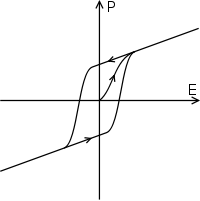
In addition to being nonlinear, ferroelectric materials demonstrate a spontaneous nonzero polarization (after entrainment, see figure) even when the applied field E is zero. The distinguishing feature of ferroelectrics is that the spontaneous polarization can be reversed by a suitably strong applied electric field in the opposite direction; the polarization is therefore dependent not only on the current electric field but also on its history, yielding a hysteresis loop. They are called ferroelectrics by analogy to ferromagnetic materials, which have spontaneous magnetization and exhibit similar hysteresis loops.
Typically, materials demonstrate ferroelectricity only below a certain phase transition temperature, called the Curie temperature (TC) and are paraelectric above this temperature: the spontaneous polarization vanishes, and the ferroelectric crystal transforms into the paraelectric state. Many ferroelectrics lose their piezoelectric properties above Tc completely, because their paraelectric phase has a centrosymmetric crystal structure.[5]
Applications
The nonlinear nature of ferroelectric materials can be used to make capacitors with tunable capacitance. Typically, a ferroelectric capacitor simply consists of a pair of electrodes sandwiching a layer of ferroelectric material. The permittivity of ferroelectrics is not only tunable but commonly also very high in absolute value, especially when close to the phase transition temperature. Because of this, ferroelectric capacitors are small in physical size compared to dielectric (non-tunable) capacitors of similar capacitance.
The spontaneous polarization of ferroelectric materials implies a hysteresis effect which can be used as a memory function, and ferroelectric capacitors are indeed used to make ferroelectric RAM[6] for computers and RFID cards. In these applications thin films of ferroelectric materials are typically used, as this allows the field required to switch the polarization to be achieved with a moderate voltage. However, when using thin films a great deal of attention needs to be paid to the interfaces, electrodes and sample quality for devices to work reliably.[7]
Ferroelectric materials are required by symmetry considerations to be also piezoelectric and pyroelectric. The combined properties of memory, piezoelectricity, and pyroelectricity make ferroelectric capacitors very useful, e.g. for sensor applications. Ferroelectric capacitors are used in medical ultrasound machines (the capacitors generate and then listen for the ultrasound ping used to image the internal organs of a body), high quality infrared cameras (the infrared image is projected onto a two dimensional array of ferroelectric capacitors capable of detecting temperature differences as small as millionths of a degree Celsius), fire sensors, sonar, vibration sensors, and even fuel injectors on diesel engines.
Another idea of recent interest is the ferroelectric tunnel junction (FTJ) in which a contact is made up by nanometer-thick ferroelectric film placed between metal electrodes.[8] The thickness of the ferroelectric layer is small enough to allow tunneling of electrons. The piezoelectric and interface effects as well as the depolarization field may lead to a giant electroresistance (GER) switching effect.
Yet another hot topic is multiferroics, where researchers are looking for ways to couple magnetic and ferroelectric ordering within a material or heterostructure; there are several recent reviews on this topic.[9]
Catalytic properties of ferroelectrics have been studied since 1952 when Parravano observed anomalies in CO oxidation rates over ferroelectric sodium and potassium niobates near the Curie temperature of these materials.[10] Surface-perpendicular component of the ferroelectric polarization can dope polarization-dependent charges on surfaces of ferroelectric materials, changing their chemistry.[11][12][13] This opens the possibility of performing catalysis beyond the limits of the Sabatier principle.[14] Sabatier principle states that the surface-adsorbates interaction has to be an optimal amount: not too weak to be inert toward the reactants and not too strong to poison the surface and avoid desorption of the products: a compromise situation.[15] This set of optimum interactions is usually referred to as "top of the volcano" in activity volcano plots.[16] On the other hand, ferroelectric polarization-dependent chemistry can offer the possibility of switching the surface—adsorbates interaction from strong adsorption to strong desorption, thus a compromise between desorption and adsorption is no longer needed.[14] Ferroelectric polarization can also act as an energy harvester.[17] Polarization can help the separation of photo-generated electron-hole pairs, leading to enhanced photocatalysis.[18] Also, due to pyroelectric and piezoelectric effects under varying temperature (heating/cooling cycles)[19][20] or varying strain (vibrations) conditions[21] extra charges can appear on the surface and drive various (electro)chemical reactions forward.
Materials
The internal electric dipoles of a ferroelectric material are coupled to the material lattice so anything that changes the lattice will change the strength of the dipoles (in other words, a change in the spontaneous polarization). The change in the spontaneous polarization results in a change in the surface charge. This can cause current flow in the case of a ferroelectric capacitor even without the presence of an external voltage across the capacitor. Two stimuli that will change the lattice dimensions of a material are force and temperature. The generation of a surface charge in response to the application of an external stress to a material is called piezoelectricity. A change in the spontaneous polarization of a material in response to a change in temperature is called pyroelectricity.
Generally, there are 230 space groups among which 32 crystalline classes can be found in crystals. There are 21 non-centrosymmetric classes, within which 20 are piezoelectric. Among the piezoelectric classes, 10 have a spontaneous electric polarization, that varies with the temperature, therefore they are pyroelectric. Among pyroelectric materials, some of them are ferroelectric.
| 32 Crystalline classes | ||||
|---|---|---|---|---|
| 21 noncentrosymmetric | 11 centrosymmetric | |||
| 20 classes piezoelectric | non piezoelectric | |||
| 10 classes pyroelectric | non pyroelectric | |||
| ferroelectric | non ferroelectric | |||
| e.g. : PbZr/TiO3, BaTiO3, PbTiO3 | e.g. : Tourmaline, ZnO, AlN | e.g. : Quartz, Langasite | ||
Ferroelectric phase transitions are often characterized as either displacive (such as BaTiO3) or order-disorder (such as NaNO2), though often phase transitions will demonstrate elements of both behaviors. In barium titanate, a typical ferroelectric of the displacive type, the transition can be understood in terms of a polarization catastrophe, in which, if an ion is displaced from equilibrium slightly, the force from the local electric fields due to the ions in the crystal increases faster than the elastic-restoring forces. This leads to an asymmetrical shift in the equilibrium ion positions and hence to a permanent dipole moment. The ionic displacement in barium titanate concerns the relative position of the titanium ion within the oxygen octahedral cage. In lead titanate, another key ferroelectric material, although the structure is rather similar to barium titanate the driving force for ferroelectricity is more complex with interactions between the lead and oxygen ions also playing an important role. In an order-disorder ferroelectric, there is a dipole moment in each unit cell, but at high temperatures they are pointing in random directions. Upon lowering the temperature and going through the phase transition, the dipoles order, all pointing in the same direction within a domain.
An important ferroelectric material for applications is lead zirconate titanate (PZT), which is part of the solid solution formed between ferroelectric lead titanate and anti-ferroelectric lead zirconate. Different compositions are used for different applications; for memory applications, PZT closer in composition to lead titanate is preferred, whereas piezoelectric applications make use of the diverging piezoelectric coefficients associated with the morphotropic phase boundary that is found close to the 50/50 composition.
Ferroelectric crystals often show several transition temperatures and domain structure hysteresis, much as do ferromagnetic crystals. The nature of the phase transition in some ferroelectric crystals is still not well understood.
In 1974 R.B. Meyer used symmetry arguments to predict ferroelectric liquid crystals,[22] and the prediction could immediately be verified by several observations of behavior connected to ferroelectricity in smectic liquid-crystal phases that are chiral and tilted. The technology allows the building of flat-screen monitors. Mass production between 1994 and 1999 was carried out by Canon. Ferroelectric liquid crystals are used in production of reflective LCoS.
In 2010 David Field found that prosaic films of chemicals such as nitrous oxide or propane exhibited ferroelectric properties. This new class of ferroelectric materials exhibit "spontelectric" properties, and may have wide-ranging applications in device and nano-technology and also influence the electrical nature of dust in the interstellar medium.
Other ferroelectric materials used include triglycine sulfate, polyvinylidene fluoride (PVDF) and lithium tantalate.[23]
It should be possible to produce materials which combine both ferroelectric and metallic properties simultaneously, at room temperature.[24] According to research published in 2018 in Nature Communications,[25] scientists were able to produce a "two-dimensional" sheet of material which was both "ferroelectric" (had a polar crystal structure) and which conducted electricity.
Theory
An introduction to Landau theory can be found here.[26] Based on Ginzburg–Landau theory, the free energy of a ferroelectric material, in the absence of an electric field and applied stress may be written as a Taylor expansion in terms of the order parameter, P. If a sixth order expansion is used (i.e. 8th order and higher terms truncated), the free energy is given by:
where Px, Py, and Pz are the components of the polarization vector in the x, y, and z directions respectively, and the coefficients, must be consistent with the crystal symmetry. To investigate domain formation and other phenomena in ferroelectrics, these equations are often used in the context of a phase field model. Typically, this involves adding a gradient term, an electrostatic term and an elastic term to the free energy. The equations are then discretized onto a grid using the finite difference method and solved subject to the constraints of Gauss's law and Linear elasticity.
In all known ferroelectrics, and . These coefficients may be obtained experimentally or from ab-initio simulations. For ferroelectrics with a first order phase transition, , whereas for a second order phase transition.
The spontaneous polarization, Ps of a ferroelectric for a cubic to tetragonal phase transition may be obtained by considering the 1D expression of the free energy which is:
This free energy has the shape of a double well potential with two free energy minima at , where Ps is the spontaneous polarization. At these two minima, the derivative of the free energy is zero, i.e.:
Since Px = 0 corresponds to a free energy maxima in the ferroelectric phase, the spontaneous polarization, Ps, is obtained from the solution of the equation:
which is:
and elimination of solutions yielding a negative square root (for either the first or second order phase transitions) gives:
If , using the same approach as above, the spontaneous polarization may be obtained as:
The hysteresis loop (Px versus Ex) may be obtained from the free energy expansion by adding another electrostatic term, Ex Px, as follows:
Plotting Ex as a function of Px and reflecting the graph about the 45 degree line gives an 'S' shaped curve. The central part of the 'S' corresponds to a free energy local maximum (since ). Elimination of this region, and connection of the top and bottom portions of the 'S' curve by vertical lines at the discontinuities gives the hysteresis loop.
See also
|
Physics
|
Lists
|
References
- Werner Känzig (1957). "Ferroelectrics and Antiferroelectrics". In Frederick Seitz; T. P. Das; David Turnbull; E. L. Hahn (eds.). Solid State Physics. 4. Academic Press. p. 5. ISBN 978-0-12-607704-9.
- M. Lines; A. Glass (1979). Principles and applications of ferroelectrics and related materials. Clarendon Press, Oxford. ISBN 978-0-19-851286-8.
- See J. Valasek (1920). "Piezoelectric and allied phenomena in Rochelle salt". Physical Review. 15 (6): 537. Bibcode:1920PhRv...15..505.. doi:10.1103/PhysRev.15.505. and J. Valasek (1921). "Piezo-Electric and Allied Phenomena in Rochelle Salt". Physical Review. 17 (4): 475. Bibcode:1921PhRv...17..475V. doi:10.1103/PhysRev.17.475. hdl:11299/179514.
- Chiang, Y. et al.: Physical Ceramics, John Wiley & Sons 1997, New York
- Safari, Ahmad (2008). Piezoelectric and acoustic materials for transducer applications. Springer Science & Business Media. p. 21. Bibcode:2008pamt.book.....S. ISBN 978-0387765402.
- J.F. Scott (2000). Ferroelectric Memories. Springer. ISBN 978-3-540-66387-4.
- M. Dawber; K.M. Rabe; J.F. Scott (2005). "Physics of thin-film ferroelectric oxides". Reviews of Modern Physics. 77 (4): 1083. arXiv:cond-mat/0503372. Bibcode:2005RvMP...77.1083D. doi:10.1103/RevModPhys.77.1083.
- M.Ye. Zhuravlev; R.F. Sabirianov; S.S. Jaswal; E.Y. Tsymbal (2005). "Giant Electroresistance in Ferroelectric Tunnel Junctions". Physical Review Letters. 94 (24): 246802–4. arXiv:cond-mat/0502109. Bibcode:2005PhRvL..94x6802Z. doi:10.1103/PhysRevLett.94.246802.
- Ramesh, R.; Spaldin, N.A (2007). "Multiferroics: Progress and prospects in thin films". Nature Materials. 6 (1): 21–9. Bibcode:2007NatMa...6...21R. doi:10.1038/nmat1805. PMID 17199122.W. Eerenstein; N.D. Mathur; J.F. Scott (2006). "Multiferroic and magnetoelectric materials". Nature. 442 (7104): 759–65. Bibcode:2006Natur.442..759E. doi:10.1038/nature05023. PMID 16915279., Spaldin, N.A.; Fiebig, M. (2005). "The renaissance of magnetoelectric multiferroics". Science. 309 (5733): 391–2. doi:10.1126/science.1113357. PMID 16020720. M. Fiebig (2005). "Revival of the magnetoelectric effect". Journal of Physics D: Applied Physics. 38 (8): R123. Bibcode:2005JPhD...38R.123F. doi:10.1088/0022-3727/38/8/R01.
- Parravano, G. (February 1952). "Ferroelectric Transitions and Heterogenous Catalysis". The Journal of Chemical Physics. 20 (2): 342–343. Bibcode:1952JChPh..20..342P. doi:10.1063/1.1700412.
- Kakekhani, Arvin; Ismail-Beigi, Sohrab; Altman, Eric I. (August 2016). "Ferroelectrics: A pathway to switchable surface chemistry and catalysis". Surface Science. 650: 302–316. Bibcode:2016SurSc.650..302K. doi:10.1016/j.susc.2015.10.055.
- Kolpak, Alexie M.; Grinberg, Ilya; Rappe, Andrew M. (2007-04-16). "Polarization Effects on the Surface Chemistry of ${\mathrm{PbTiO}}_{3}$-Supported Pt Films". Physical Review Letters. 98 (16): 166101. doi:10.1103/PhysRevLett.98.166101. PMID 17501432.
- Yun, Yang; Altman, Eric I. (December 2007). "Using Ferroelectric Poling to Change Adsorption on Oxide Surfaces". Journal of the American Chemical Society. 129 (50): 15684–15689. doi:10.1021/ja0762644. PMID 18034485.
- Kakekhani, Arvin; Ismail-Beigi, Sohrab (29 June 2015). "Ferroelectric-Based Catalysis: Switchable Surface Chemistry". ACS Catalysis. 5 (8): 4537–4545. Bibcode:2015APS..MARY26011K. doi:10.1021/acscatal.5b00507.
- Laursen, Anders B.; Man, Isabela Costinela; Trinhammer, Ole L.; Rossmeisl, Jan; Dahl, Søren (December 2011). "The Sabatier Principle Illustrated by Catalytic H2O2 Decomposition on Metal Surfaces". Journal of Chemical Education. 88 (12): 1711–1715. Bibcode:2011JChEd..88.1711L. doi:10.1021/ed101010x.
- Seh, Zhi Wei; Kibsgaard, Jakob; Dickens, Colin F.; Chorkendorff, Ib; Nørskov, Jens K.; Jaramillo, Thomas F. (13 January 2017). "Combining theory and experiment in electrocatalysis: Insights into materials design" (PDF). Science. 355 (6321): eaad4998. doi:10.1126/science.aad4998. PMID 28082532.
- Zhang, Yan; Xie, Mengying; Adamaki, Vana; Khanbareh, Hamideh; Bowen, Chris R. (2017). "Control of electro-chemical processes using energy harvesting materials and devices". Chemical Society Reviews. 46 (24): 7757–7786. doi:10.1039/c7cs00387k. PMID 29125613.
- Fang, Liang; You, Lu; Liu, Jun-Ming (2018). "Ferroelectrics in Photocatalysis". Ferroelectric Materials for Energy Applications. pp. 265–309. doi:10.1002/9783527807505.ch9. ISBN 9783527807505.
- Benke, Annegret; Mehner, Erik; Rosenkranz, Marco; Dmitrieva, Evgenia; Leisegang, Tilmann; Stöcker, Hartmut; Pompe, Wolfgang; Meyer, Dirk C. (30 July 2015). "Pyroelectrically Driven •OH Generation by Barium Titanate and Palladium Nanoparticles". The Journal of Physical Chemistry C. 119 (32): 18278–18286. doi:10.1021/acs.jpcc.5b04589.
- Kakekhani, Arvin; Ismail-Beigi, Sohrab (2016). "Ferroelectric oxide surface chemistry: water splitting via pyroelectricity". Journal of Materials Chemistry A. 4 (14): 5235–5246. doi:10.1039/C6TA00513F.
- Starr, Matthew B.; Shi, Jian; Wang, Xudong (11 June 2012). "Piezopotential-Driven Redox Reactions at the Surface of Piezoelectric Materials". Angewandte Chemie International Edition. 51 (24): 5962–5966. doi:10.1002/anie.201201424. PMID 22556008.
- Clark, Noel A.; Lagerwall, Sven T. (June 1980). "Submicrosecond bistable electro‐optic switching in liquid crystals". Applied Physics Letters. 36 (11): 899–901. Bibcode:1980ApPhL..36..899C. doi:10.1063/1.91359.
- Aggarwal, M.D.; A.K. Batra; P. Guggilla; M.E. Edwards; B.G. Penn; J.R. Currie Jr. (March 2010). "Pyroelectric Materials for Uncooled Infrared Detectors: Processing, Properties, and Applications" (PDF). NASA. p. 3. Retrieved 26 July 2013.
- https://www.rutgers.edu/news/rutgers-physicists-create-new-class-2d-artificial-materials
- Cao, Yanwei; Wang, Zhen; Park, Se Young; Yuan, Yakun; Liu, Xiaoran; Nikitin, Sergey M.; Akamatsu, Hirofumi; Kareev, M.; Middey, S.; Meyers, D.; Thompson, P.; Ryan, P. J.; Shafer, Padraic; N’Diaye, A.; Arenholz, E.; Gopalan, Venkatraman; Zhu, Yimei; Rabe, Karin M.; Chakhalian, J. (18 April 2018). "Artificial two-dimensional polar metal at room temperature". Nature Communications. 9 (1): 1547. arXiv:1804.05487. Bibcode:2018NatCo...9.1547C. doi:10.1038/s41467-018-03964-9. PMC 5906683. PMID 29670098.
- P. Chandra; P.B. Littlewood (2006). "A Landau Primer for Ferroelectrics". arXiv:cond-mat/0609347.
Further reading
- A. S. Sidorkin (2006). Domain Structure in Ferroelectrics and Related Materials. Cambridge University Press. ISBN 978-1-904602-14-9.
- Karin M Rabe; Jean-Marc Triscone; Charles H Ahn (2007). Physics of Ferroelectrics: A modern perspective. Springer. ISBN 978-3-540-34591-6.
- Julio A. Gonzalo (2006). Effective Field Approach to Phase Transitions and Some Applications to Ferroelectrics. World Scientific. ISBN 978-981-256-875-5.