Esophageal achalasia
Esophageal achalasia, often referred to simply as achalasia, is a failure of smooth muscle fibers to relax, which can cause the lower esophageal sphincter to remain closed. Without a modifier, "achalasia" usually refers to achalasia of the esophagus. Achalasia can happen at various points along the gastrointestinal tract; achalasia of the rectum, for instance, may occur in Hirschsprung's disease.
| Esophageal achalasia | |
|---|---|
| Other names | Achalasia cardiae, cardiospasm, esophageal aperistalsis |
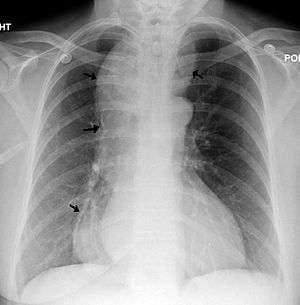 | |
| A chest X-ray showing achalasia ( arrows point to the outline of the massively dilated esophagus ) | |
| Pronunciation |
|
| Specialty | Thoracic surgery, general surgery |
| Deaths | 79 |
Esophageal achalasia is an esophageal motility disorder involving the smooth muscle layer of the esophagus and the lower esophageal sphincter (LES).[1] It is characterized by incomplete LES relaxation, increased LES tone, and lack of peristalsis of the esophagus (inability of smooth muscle to move food down the esophagus) in the absence of other explanations like cancer or fibrosis.[2][3][4]
Achalasia is characterized by difficulty in swallowing, regurgitation, and sometimes chest pain. Diagnosis is reached with esophageal manometry and barium swallow radiographic studies. Various treatments are available, although none cures the condition. Certain medications or Botox may be used in some cases, but more permanent relief is brought by esophageal dilatation and surgical cleaving of the muscle (Heller myotomy).
The most common form is primary achalasia, which has no known underlying cause. It is due to the failure of distal esophageal inhibitory neurons. However, a small proportion occurs secondary to other conditions, such as esophageal cancer, Chagas disease (an infectious disease common in South America) or Triple-A syndrome.[5] Achalasia affects about one person in 100,000 per year.[5][6] There is no gender predominance for the occurrence of disease.[7] The term is from a- + -chalasia "no relaxation."
Achalasia can also manifest alongside other diseases as a rare syndrome such as achalasia microcephaly.[8]
Signs and symptoms
The main symptoms of achalasia are dysphagia (difficulty in swallowing), regurgitation of undigested food, chest pain behind the sternum, and weight loss.[9] Dysphagia tends to become progressively worse over time and to involve both fluids and solids. Some people may also experience coughing when lying in a horizontal position. The chest pain experienced, also known as cardiospasm and non-cardiac chest pain can often be mistaken for a heart attack. It can be extremely painful in some sufferers. Food and liquid, including saliva, are retained in the esophagus and may be inhaled into the lungs (aspiration).
Mechanism
The cause of most cases of achalasia is unknown.[10] LES pressure and relaxation are regulated by excitatory (e.g., acetylcholine, substance P) and inhibitory (e.g., nitric oxide, vasoactive intestinal peptide) neurotransmitters. People with achalasia lack noradrenergic, noncholinergic, inhibitory ganglion cells, causing an imbalance in excitatory and inhibitory neurotransmission. The result is a hypertensive nonrelaxed esophageal sphincter.[11]
Autopsy and myotomy specimens have, on histological examination, shown an inflammatory response consisting of CD3/CD8-positive cytotoxic T lymphocytes, variable numbers of eosinophils and mast cells, loss of ganglion cells, and neurofibrosis; these events appear to occur early in achalasia. Thus, it seems there is an autoimmune context to achalasia, most likely caused by viral triggers. Other studies suggest hereditary, neurodegenerative, genetic and infective contributions.[12]
Diagnosis
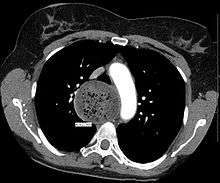
Due to the similarity of symptoms, achalasia can be mistaken for more common disorders such as gastroesophageal reflux disease (GERD), hiatus hernia, and even psychosomatic disorders. Specific tests for achalasia are barium swallow and esophageal manometry. In addition, endoscopy of the esophagus, stomach, and duodenum (esophagogastroduodenoscopy or EGD), with or without endoscopic ultrasound, is typically performed to rule out the possibility of cancer.[5] The internal tissue of the esophagus generally appears normal in endoscopy, although a "pop" may be observed as the scope is passed through the non-relaxing lower esophageal sphincter with some difficulty, and food debris may be found above the LES.
Barium swallow
The patient swallows a barium solution, with continuous fluoroscopy (X-ray recording) to observe the flow of the fluid through the esophagus. Normal peristaltic movement of the esophagus is not seen. There is acute tapering at the lower esophageal sphincter and narrowing at the gastro-esophageal junction, producing a "bird's beak" or "rat's tail" appearance. The esophagus above the narrowing is often dilated (enlarged) to varying degrees as the esophagus is gradually stretched over time.[5] An air-fluid margin is often seen over the barium column due to the lack of peristalsis. A five-minutes timed barium swallow can provide a useful benchmark to measure the effectiveness of treatment.
Esophageal manometry
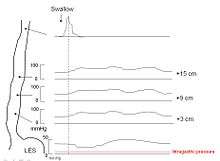
Because of its sensitivity, manometry (esophageal motility study) is considered the key test for establishing the diagnosis. A catheter (thin tube) is inserted through the nose, and the patient is instructed to swallow several times. The probe measures muscle contractions in different parts of the esophagus during the act of swallowing. Manometry reveals failure of the LES to relax with swallowing and lack of functional peristalsis in the smooth muscle esophagus.[5]
Characteristic manometric findings are:
- Lower esophageal sphincter (LES) fails to relax upon wet swallow (<75% relaxation)
- Pressure of LES <26 mm Hg is normal,>100 is considered achalasia, > 200 is nutcracker achalasia.
- Aperistalsis in esophageal body
- Relative increase in intra-esophageal pressure as compared with intra-gastric pressure
Biopsy
Biopsy, the removal of a tissue sample during endoscopy, is not typically necessary in achalasia but if performed shows hypertrophied musculature and absence of certain nerve cells of the myenteric plexus, a network of nerve fibers that controls esophageal peristalsis.[13] It is not possible to diagnose achalasia by means of biopsy alone.[14]
Treatment
Sublingual nifedipine significantly improves outcomes in 75% of people with mild or moderate disease. It was classically considered that surgical myotomy provided greater benefit than either botulinum toxin or dilation in those who fail medical management.[15] However, a recent randomized controlled trial found pneumatic dilation to be non-inferior to laparoscopic Heller myotomy.[16]
Lifestyle changes
Both before and after treatment, achalasia patients may need to eat slowly, chew very well, drink plenty of water with meals, and avoid eating near bedtime. Raising the head off the bed or sleeping with a wedge pillow promotes emptying of the esophagus by gravity. After surgery or pneumatic dilatation, proton pump inhibitors are required to prevent reflux damage by inhibiting gastric acid secretion, and foods that can aggravate reflux, including ketchup, citrus, chocolate, alcohol, and caffeine, may need to be avoided.
Medication
Drugs that reduce LES pressure are useful. These include calcium channel blockers such as nifedipine[15] and nitrates such as isosorbide dinitrate and nitroglycerin. However, many patients experience unpleasant side effects such as headache and swollen feet, and these medications often stop helping after several months.
Botulinum toxin (Botox) may be injected into the lower esophageal sphincter to paralyze the muscles holding it shut. As in the case of cosmetic Botox, the effect is only temporary and lasts about 6 months. Botox injections cause scarring in the sphincter which may increase the difficulty of later Heller myotomy. This therapy is recommended only for patients who cannot risk surgery, such as elderly people in poor health.[5] Pneumatic dilatation has a better long term effectiveness than botox.[17]
Pneumatic dilatation
In balloon (pneumatic) dilation or dilatation, the muscle fibers are stretched and slightly torn by forceful inflation of a balloon placed inside the lower esophageal sphincter. There is always a small risk of a perforation which requires immediate surgical repair. Pneumatic dilatation causes some scarring which may increase the difficulty of Heller myotomy if the surgery is needed later. Gastroesophageal reflux (GERD) occurs after pneumatic dilatation in some patients. Pneumatic dilatation is most effective in the long-term on patients over the age of 40; the benefits tend to be shorter-lived in younger patients. It may need to be repeated with larger balloons for maximum effectiveness.[6]
Surgery
Heller myotomy helps 90% of achalasia patients. It can usually be performed by a keyhole approach or laparoscopically.[18] The myotomy is a lengthwise cut along the esophagus, starting above the LES and extending down onto the stomach a little way. The esophagus is made of several layers, and the myotomy cuts only through the outside muscle layers which are squeezing it shut, leaving the inner mucosal layer intact. A partial fundoplication or "wrap" is generally added in order to prevent excessive reflux, which can cause serious damage to the esophagus over time. After surgery, patients should keep to a soft diet for several weeks to a month, avoiding foods that can aggravate reflux.
The most recommended fundoplication to complement Heller myotomy is Dor fundoplication, which consists of a 180- to 200-degree anterior wrap around the esophagus. It provides excellent results as compared to Nissen's fundoplication, which is associated with higher incidence of postoperative dysphagia.[19]
The shortcoming of laparoscopic esophageal myotomy is the need for a fundoplication. On the one hand, the myotomy opens the esophagus, while on the other hand, the fundoplication causes an obstruction. Recent understanding of the gastroesophageal antireflux barrier/valve has shed light on the reason for the occurrence of reflux following myotomy. The gastroesophageal valve is the result of infolding of the esophagus into the stomach at the esophageal hiatus. This infolding creates a valve which extends from 7 o'clock to 4 o'clock (270 degrees) around the circumference of the esophagus. Laparoscopic myotomy cuts the muscle at the 12 o'clock position, resulting in incompetence of the valve and reflux. Recent robotic laparoscopic series have attempted a myotomy at the 5 o'clock position on the esophagus away from the valve. The robotic lateral esophageal myotomy preserves the esophageal valve and does not result in reflux, thereby obviating the need for a fundoplication. The robotic lateral esophageal myotomy has had the best results to date in terms of ability to eat without reflux.
Endoscopic myotomy
A new endoscopic therapy for achalasia management was developed in 2008 in Japan.[20] Per-oral endoscopic myotomy or POEM is a minimally invasive type of natural orifice transluminal endoscopic surgery that follows the same principle as the Heller myotomy. A tiny incision is made on the esophageal mucosa through which an endoscope is inserted. The innermost circular muscle layer of the esophagus is divided and extended through the LES until about 2 cm into the gastric muscle. Since this procedure is performed entirely through the patient's mouth, there are no visible scars on the patient's body.
Patients usually spend about 1–4 days in the hospital and are discharged after satisfactory examinations. Patients are discharged on full diet and generally able to return to work and full activity immediately upon discharge.[21] Major complications are rare after POEM and are generally managed without intervention. Long term patient satisfaction is similar following POEM compared to standard laparoscopic Heller myotomy.[22]
POEM has been performed on over 1,200 patients in Japan and is becoming increasingly popular internationally as a first-line therapy in patients with achalasia.[23]
Monitoring
Even after successful treatment of achalasia, swallowing may still deteriorate over time. The esophagus should be checked every year or two with a timed barium swallow because some may need pneumatic dilatations, a repeat myotomy, or even esophagectomy after many years. In addition, some physicians recommend pH testing and endoscopy to check for reflux damage, which may lead to a premalignant condition known as Barrett's esophagus or a stricture if untreated.
Epidemiology
Incidence of achalasia has risen to approximately 1.6 per 100,000 in some populations. Disease affects mostly adults between ages 30s and 50s.[24]
References
- Park W, Vaezi M (2005). "Etiology and pathogenesis of achalasia: the current understanding". American Journal of Gastroenterology. 100 (6): 1404–1414. PMID 15929777.
- Spechler SJ, Castell DO (July 2001). "Classification of oesophageal motility abnormalities". Gut. 49 (1): 145–151. doi:10.1136/gut.49.1.145. PMC 1728354. PMID 11413123.
- Pandolfino JE, Kahrilas PJ (January 2005). "AGA technical review on the clinical use of esophageal manometry". Gastroenterology. 128 (1): 209–224. doi:10.1053/j.gastro.2004.11.008. PMID 15633138.
- Pandolfino, JE; Gawron, AJ (2015). "2015 JAMA Review of Achalasia". Journal of the American Medical Association. 313 (18): 1841–1852. doi:10.1001/jama.2015.2996. PMID 25965233.
- Spiess AE, Kahrilas PJ (August 1998). "Treating achalasia: from whalebone to laparoscope". Journal of the American Medical Association. 280 (7): 638–642. doi:10.1001/jama.280.7.638. PMID 9718057.
- Lake JM, Wong RK (September 2006). "Review article: the management of achalasia - a comparison of different treatment modalities". Alimentary Pharmacology and Therapeutics. 24 (6): 909–918. doi:10.1111/j.1365-2036.2006.03079.x. PMID 16948803.
- Francis, DL; Katzka, DA (August 2010). "Achalasia: update on the disease and its treatment". Gastroenterology. 139 (2): 369–374. doi:10.1053/j.gastro.2010.06.024. PMID 20600038.
- Gockel, Henning R.; Schumacher, Johannes; Gockel, Ines; Lang, Hauke; Haaf, Thomas; Nöthen, Markus M. (2010-08-11). "Achalasia: will genetic studies provide insights?". Human Genetics. 128 (4): 353–364. doi:10.1007/s00439-010-0874-8. ISSN 0340-6717. PMID 20700745.
- Dughera, L; Cassolino, P; Cisarò, F; Chiaverina, M (September 2008). "Achalasia". Minerva Gastroenterologica e Dietologica. 54 (3): 277–285. PMID 18614976.
- Cheatham, JG; Wong, RK (June 2011). "Current approach to the treatment of achalasia". Current Gastroenterology Reports. 13 (3): 219–225. doi:10.1007/s11894-011-0190-z. PMID 21424734.
- Achalasia at eMedicine
- Chuah, SK; Hsu, PI; Wu, KL; Wu, DC; Tai, WC; Changchien, CS (14 April 2012). "2011 update on esophageal achalasia" (PDF). World Journal of Gastroenterology. 18 (14): 1573–1578. doi:10.3748/wjg.v18.i14.1573. PMC 3325522. PMID 22529685. Archived from the original (PDF) on 26 June 2015.
- Emanuel Rubin; Fred Gorstein; Raphael Rubin; Roland Schwarting; David Strayer (2001). Rubin's Pathology - clinicopathological foundations of medicine. Maryland: Lippincott Williams & Wilkins. p. 665. ISBN 978-0-7817-4733-2.
- Döhla, M; Leichauer, K; Gockel, I; Niebisch, S; Thieme, R; Lundell, L; Schumacher, J; Becker, J; Rieker, RJ; Hartmann, A; Vieth, M; Veits, L (March 2019). "Characterization of esophageal inflammation in patients with achalasia. A retrospective immunohistochemical study". Human Pathology. 85: 228–234. doi:10.1016/j.humpath.2018.11.006. PMID 30502378.
- Wang L, Li YM, Li L (November 2009). "Meta-analysis of randomized and controlled treatment trials for achalasia". Digestive Diseases and Sciences. 54 (11): 2303–2311. doi:10.1007/s10620-008-0637-8. PMID 19107596.
- Boeckxstaens, GE; Annese, V; Bruley des Varannes, S; Chaussade, S; Costantini, M; Cuttitta, A; Elizalde, JI; Fumagalli U; Gaudric, M; Rohof, WO; Smout, AJ; Tack, J; Zwinderman, AH; Zaninotto, G; Busch, OR (12 May 2011). "Pneumatic Dilation versus Laparoscopic Heller Myotomy for Idiopathic Achalasia". New England Journal of Medicine. 364 (3): 1807–1816. doi:10.1056/nejmoa1010502. PMID 21561346.
- Leyden, JE; Moss, AC; MacMathuna, P (8 December 2014). "Endoscopic pneumatic dilation versus botulinum toxin injection in the management of primary achalasia". The Cochrane Database of Systematic Reviews. 12 (12): CD005046. doi:10.1002/14651858.CD005046.pub3. PMID 25485740.
- Deb S, Deschamps C, Cassivi SD, et al. (2005). "Laparoscopic esophageal myotomy for achalasia: factors affecting functional results". Annals of Thoracic Surgery. 80 (4): 1191–1195. doi:10.1016/j.athoracsur.2005.04.008. PMID 16181839.
- Rebecchi F, Giaccone C, Farinella E, Campaci R, Morino M (December 2008). "Randomized controlled trial of laparoscopic Heller myotomy plus Dor fundoplication versus Nissen fundoplication for achalasia: long-term results". Annals of Surgery. 248 (6): 1023–1030. doi:10.1097/SLA.0b013e318190a776. PMID 19092347.
- Inoue, H.; Minami, H.; Kobayashi, Y.; Sato, Y.; Kaga, M.; Suzuki, M.; Satodate, H.; Odaka, N.; Itoh, H. (2010-04-01). "Peroral endoscopic myotomy (POEM) for esophageal achalasia". Endoscopy. 42 (4): 265–271. doi:10.1055/s-0029-1244080. ISSN 0013-726X. PMID 20354937.
- Inoue, Haruhiro; Sato, Hiroki; Ikeda, Haruo; Onimaru, Manabu; Sato, Chiaki; Minami, Hitomi; Yokomichi, Hiroshi; Kobayashi, Yasutoshi; Grimes, Kevin L. (2015-08-01). "Per-Oral Endoscopic Myotomy: A Series of 500 Patients". Journal of the American College of Surgeons. 221 (2): 256–264. doi:10.1016/j.jamcollsurg.2015.03.057. ISSN 1072-7515. PMID 26206634.
- Bechara, Robert; Onimaru, Manabu; Ikeda, Haru; Inoue, Haruhiro (2016-08-01). "Per-oral endoscopic myotomy, 1000 cases later: pearls, pitfalls, and practical considerations". Gastrointestinal Endoscopy. 84 (2): 330–338. doi:10.1016/j.gie.2016.03.1469. ISSN 0016-5107. PMID 27020899.
- Tuason, Joshua; Inoue, Haruhiro (2017-04-01). "Current status of achalasia management: a review on diagnosis and treatment". Journal of Gastroenterology. 52 (4): 401–406. doi:10.1007/s00535-017-1314-5. ISSN 0944-1174. PMID 28188367.
- O'Neill OM, Johnston BT, Coleman HG (21 Sep 2013). "Achalasia: A review of clinical diagnosis, epidemiology, treatment and outcomes". World J Gastroenterol. 19 (35): 5806–5812. doi:10.3748/wjg.v19.i35.5806. PMC 3793135. PMID 24124325.
External links
| Classification | |
|---|---|
| External resources |
- Esophageal achalasia at Curlie
- U.S. Society for Surgery of the Alimentary Tract - Achalasia treatment guidelines
- Radiology of Achalasia on YouTube