Epiblast
In amniote animal embryology, the epiblast (also known as the primitive ectoderm) is one of two distinct layers arising from the inner cell mass in the mammalian blastocyst or from the blastodisc in reptiles and birds. It derives the embryo proper through its differentiation into the three primary germ layers, ectoderm, mesoderm and endoderm, during gastrulation. The amnionic ectoderm and extraembryonic mesoderm also originate from the epiblast.
| Epiblast | |
|---|---|
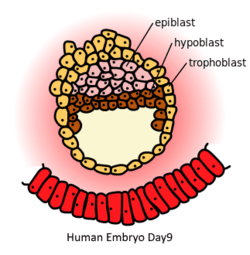 Human Embryo in day 9. Epiblast (pink) is on the top of hypoblast (brown) | |
| Details | |
| Carnegie stage | 3 |
| Days | 8 |
| Precursor | inner cell mass |
| Gives rise to | ectoderm, mesoderm, endoderm |
| Identifiers | |
| Latin | epiblastus |
| TE | E5.0.2.2.1.0.1 |
| Anatomical terminology | |
The other layer of the inner cell mass, the hypoblast, gives rise to the yolk sac, which in turn gives rise to the chorion.
Mammals
In mammalian embryogenesis, differentiation and segregation of cells composing the inner cell mass of the blastocyst yields two distinct layers—the epiblast ("primitive ectoderm") and the hypoblast ("primitive endoderm"). While the cuboidal hypoblast cells delaminate ventrally, away from the embryonic pole, to line the blastocoele, the remaining cells of the inner cell mass, situated between the hypoblast and the polar trophoblast, become the epiblast and comprise columnar cells.
In the mouse, primordial germ cells are specified from epiblast cells.[1] This specification is accompanied by extensive epigenetic reprogramming that involves global DNA demethylation, chromatin reorganization and imprint erasure leading to totipotency.[1] The DNA base excision repair pathway has a central role in the process of genome-wide demethylation.[2]
Upon commencement of gastrulation, the primitive streak, a visible, morphological groove, appears on the posterior epiblast and orients along the anterior-posterior embryo axis. Initiated by signals from the underlying hypoblast, formation of the primitive streak is predicated on epiblast cell migration, mediated by Nodal, from the lateral-posterior regions of the epiblast to the center midline.[3] The primitive knot is situated at the anterior end of the primitive streak and serves as the organizer for gastrulation, determining epiblast cell fate by inducing the differentiation of migrating epiblast cells during gastrulation.
During gastrulation, migrating epiblast cells undergo epithelial-mesenchymal transition in order to lose cell-cell adhesion (E-cadherin), delaminate from the epiblast layer and migrate over the dorsal surface of the epiblast then down through the primitive streak. The first wave of epiblast cells to invaginate through the primitive streak invades and displaces the hypoblast to become the embryonic endoderm. The mesoderm layer is established next as migrating epiblast cells move through the primitive streak then spread out within the space between the endoderm and remaining epiblast, which once the mesoderm layer has formed ultimately becomes the definitive ectoderm. The process of gastrulation results in a trilaminar germ disc, consisting of the ectoderm, mesoderm and endoderm layers.
Epiblast diversity
Epiblasts exhibit diverse structure across species as a result of early embryo morphogenesis. The human epiblast assumes a disc shape, conforming to the embryonic disc morphology; whereas, the mouse epiblast develops in a cup shape within the cylindrical embryo.
Birds
Gastrulation occurs in the epiblast of avian embryos. A local thickening of the epiblast, known as Koller's sickle, is key in inducing the primitive streak, the structure through which gastrulation occurs.[4]
See also
- Embryogenesis
- Human embryogenesis
- Hypoblast
References
- Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA (January 2013). "Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine". Science. 339 (6118): 448–52. doi:10.1126/science.1229277. PMC 3847602. PMID 23223451.
- Hajkova P, Jeffries SJ, Lee C, Miller N, Jackson SP, Surani MA (July 2010). "Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway". Science. 329 (5987): 78–82. doi:10.1126/science.1187945. PMC 3863715. PMID 20595612.
- Shen MM. Nodal signaling: developmental roles and regulation. Development 2007; 134(6): 1023-1034.
- Gilbert SF. Developmental Biology. 10th edition. Sunderland (MA): Sinauer Associates; 2014. Early Development in Birds. Print
External links
- http://www.embryology.ch/allemand/iperiodembry/carnegie02.html
- http://www.med.umich.edu/lrc/coursepages/M1/embryology/embryo/04secondweek.htm
- https://web.archive.org/web/20080103050509/http://isc.temple.edu/marino/embryology/EMBII97/sld005.htm