Eggshell
An eggshell is the outer covering of a hard-shelled egg and of some forms of eggs with soft outer coats.

Diversity
Worm eggs
Nematode eggs present a three layered structure: an External Vitellin Layer, a Chitin Layer that confers mechanical resistance and an internal Lipid-rich layer that makes the egg chamber impermeable. [1]
Insect eggs

Insects and other arthropods lay a large variety of styles and shapes of eggs. Some of them have gelatinous or skin-like coverings, others have hard eggshells. Softer shells are mostly protein. It may be fibrous or quite liquid. Some arthropod eggs do not actually have shells, rather, their outer covering is actually the outermost embryonic membrane, the choroid, which serves to protect inner layers. The choroid itself can be a complex structure, and it may have different layers within it. It may have an outermost layer called an exochorion. Eggs which must survive in dry conditions usually have hard eggshells, made mostly of dehydrated or mineralized proteins with pore systems to allow respiration. Arthropod eggs can have extensive ornamentation on their outer surfaces.
Fish, amphibian and reptile eggs
Fish and amphibians generally lay eggs which are surrounded by the extraembryonic membranes but do not develop a shell, hard or soft, around these membranes. Some fish and amphibian eggs have thick, leathery coats, especially if they must withstand physical force or desiccation. These types of eggs can also be very small and fragile.
While many reptiles lay eggs with flexible, calcified eggshells, there are some that lay hard eggs. Eggs laid by snakes generally have leathery shells which often adhere to one another. Depending on the species, turtles and tortoises lay hard or soft eggs. Several species lay eggs which are nearly indistinguishable from bird eggs.
Bird eggs
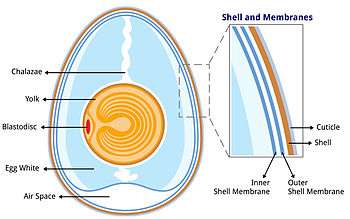
The bird egg is a fertilized gamete (or, in the case of some birds, such as chickens, possibly unfertilized) located on the yolk surface and surrounded by albumen, or egg white. The albumen in turn is surrounded by two shell membranes (inner and outer membranes) and then the eggshell. The chicken eggshell is 95-97%[2] calcium carbonate crystals, which are stabilized by a protein matrix.[3][4][2] Without the protein, the crystal structure would be too brittle to keep its form and the organic matrix is thought to have a role in deposition of calcium during the mineralization process.[5][6][7] The structure and composition of the avian eggshell serves to protect the egg against damage and microbial contamination, prevention of desiccation, regulation of gas and water exchange for the growing embryo, and provides calcium for embryogenesis. Eggshell formation requires gram amounts of calcium being deposited within hours, which must be supplied via the hen's diet.[2]

The fibrous chicken shell membranes are added in the proximal (white) isthmus of the oviduct.[2] In the distal (red) isthmus mammillae or mammillary knobs are deposited on the surface of the outer membrane in a regular array pattern.[8][9] The mammillae are proteoglycan-rich and are thought to control calcification. In the shell gland (similar to a mammalian uterus), mineralization starts at the mammillae. The shell gland fluid contains very high levels of calcium and hydrogen carbonate. The thick calcified layer of the eggshell forms in columns from the mammillae structures, and is known as the palisade layer. Between these palisade columns are narrow pores that traverse the eggshell and allow gaseous exchange. The cuticle forms the final, outer layer of the eggshell.[10]
While the bulk of eggshell is made of calcium carbonate, it is now thought that the protein matrix has an important role to play in eggshell strength.[11] These proteins affect crystallization, which in turn affects the eggshell structure. Moreover, the concentration of eggshell proteins decreases over the life of the laying hen, as does eggshell strength.
In an average laying hen, the process of shell formation takes around 20 hours. Pigmentation is added to the shell by papillae lining the oviduct, coloring it any of a variety of colors and patterns depending on species. Since eggs are usually laid blunt end first, that end is subjected to most pressure during its passage and consequently shows the most color.
As they contain mainly calcium carbonate, bird eggshells dissolve in various acids, including the vinegar used in cooking. While dissolving, the calcium carbonate in an eggshell reacts with the acid to form carbon dioxide.[12]
Environmental issues
The US food industry generates 150,000 tons of shell waste per year.[13] The disposal methods for waste eggshells are 26.6% as fertilizer, 21.1% as animal feed ingredients, 26.3% discarded in municipal dumps, and 15.8% used in other ways.[14] Many landfills are unwilling to take the waste because the shells and the attached membrane attract vermin. Together with the calcium carbonate eggshell and protein-rich membrane are useless.[15] Recent inventions have allowed for the egg cracking industry to separate the eggshell from the eggshell membrane. The eggshell is mostly made up of calcium carbonate and the membrane is valuable protein. When separated both products have an array of uses.
Mammal eggs
Monotremes, egg-laying mammals, lay soft-shelled eggs similar to those of reptiles. The shell is deposited on the egg in layers within the uterus. The egg can take up fluids and grow in size during this process, and the final, most rigid layer is not added until the egg is full-size.
Use
Eggshell waste is fundamentally composed of calcium carbonate, and has the potential to be used as raw material in the production of lime.[18] Recently, researchers have utilized chicken eggshells as a biofiller with a conducting polymer to enhance its sensing properties. Typically, eggshells were used as biofiller in polyaniline matrix to detect ammonia gas. The optimum ratio between eggshells and polyaniline could enhance this sensor measurement.[19]
See also
- Eggshell skull rule, in tort law
- Walk on eggshells, an idiom in the English language
- Eggshell membrane, a dietary supplement
References
- Benenati G, Penkov S, Müller-Reichert T, Entchev EV, Kurzchalia TV (May–Jun 2009). "Two cytochrome P450s in Caenorhabditis elegans are essential for the organization of eggshell, correct execution of meiosis and the polarization of embryo". Mech Dev. 126 (5–6): 382–93. doi:10.1016/j.mod.2009.02.001. PMID 19368796.
- Hunton, P (2005). "Research on eggshell structure and quality: an historical overview". Revista Brasileira de Ciência Avícola. 7 (2): 67–71. doi:10.1590/S1516-635X2005000200001.
- Arias, J. L.; Fernandez, M. S. (2001). "Role of extracellular matrix molecules in shell formation and structure". World's Poultry Science Journal. 57 (4): 349–357. doi:10.1079/WPS20010024.
- Nys, Yves; Gautron, Joël; Garcia-Ruiz, Juan M.; Hincke, Maxwell T. (2004). "Avian eggshell mineralization: biochemical and functional characterization of matrix proteins". Comptes Rendus Palevol. 3 (6–7): 549–62. doi:10.1016/j.crpv.2004.08.002.
- Romanoff, A.L., A.J. Romanoff (1949) The avian egg. New York, Wiley.
- Burley, R.W., D.V. Vadehra (1989) The Avian Egg: Chemistry and Biology. New York, Wiley.
- Lavelin, I; Meiri, N; Pines, M (2000). "New insight in eggshell formation". Poultry Science. 79 (7): 1014–7. CiteSeerX 10.1.1.335.6360. doi:10.1093/ps/79.7.1014. PMID 10901204.
- Wyburn, GM; Johnston, HS; Draper, MH; Davidson, MF (1973). "The ultrastructure of the shell forming region of the oviduct and the development of the shell of Gallus domesticus". Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 58 (2): 143–51. PMID 4487964.
- Fernandez, MS; Araya, M; Arias, JL (1997). "Eggshells are shaped by a precise spatio-temporal arrangement of sequentially deposited macromolecules". Matrix Biology. 16 (1): 13–20. doi:10.1016/s0945-053x(97)90112-8. PMID 9181550.
- "The Egg-Shell Microstructure Studied by Powder Diffraction". Xray.cz. Retrieved 2012-10-16.
- http://ict.udg.co.cu/FTPDocumentos/Literatura%20Cientifica/Maestria%20Nutricion%20Animal/6.%20EVENTOS%20RELEVANTES/XVII%20Congreso%20Avicultura/confs/hunton1.htm. Retrieved February 2, 2011. Missing or empty
|title=(help) - "Q & A: Eggshells in Vinegar - What happened? | Department of Physics | University of Illinois at Urbana-Champaign". Van.physics.illinois.edu. 2007-10-22. Retrieved 2012-10-16.
- Hecht J: Eggshells break into collagen market. New Scientist 1999, 161:6-6.
- Daengprok W, Garnjanagoonchorn W, Mine Y: Fermented pork sausage fortified with commercial or hen eggshell calcium lactate. Meat Science 2002, 62:199-204.
- Wei Z; Li B; Xu C (2009). "Application of waste eggshell as low-cost solid catalyst for biodiesel production". Bioresource Technology. 100 (11): 2883–2885. doi:10.1016/j.biortech.2008.12.039. PMID 19201602.
- "What is Egg Shell Quality and How to Preserve It". Ag.ansc.purdue.edu. Archived from the original on 2012-12-08. Retrieved 2012-10-16.
- Nys, Yves; Gautron, Joël; Garcia-Ruiz, Juan M.; Hincke, Maxwell T. (2004). "Avian eggshell mineralization: biochemical and functional characterization of matrix proteins" (PDF). Comptes Rendus Palevol. 3 (6–7): 549–562. doi:10.1016/j.crpv.2004.08.002.
- Ferraz, Eduardo; Gamelas, José A. F.; Coroado, João; Monteiro, Carlos; Rocha, Fernando (2018-09-03). "Eggshell waste to produce building lime: calcium oxide reactivity, industrial, environmental and economic implications". Materials and Structures. 51 (5). doi:10.1617/s11527-018-1243-7. ISSN 1359-5997.
- N A Mazlan, J M Sapari, K P Sambasevam, Synthesis and fabrication of polyaniline/eggshell composite in ammonia detection, Journal of Metals, Materials and Minerals, Vol 30, No. 2, 50-57 (2020).https://ojs.materialsconnex.com/index.php/jmmm/article/view/649
Further reading
- Kilner, R. M. (2006). "The evolution of egg colour and patterning in birds". Biological Reviews. 81 (3): 383–406. CiteSeerX 10.1.1.565.8957. doi:10.1017/S1464793106007044. PMID 16740199.
