EcoRI
EcoRI (pronounced "eco R one") is a restriction endonuclease enzyme isolated from species E. coli. The Eco part of the enzyme's name originates from the species from which it was isolated, while the R represents the particular strain, in this case RY13. The last part of its name, the I, denotes that it was the first enzyme isolated from this strain. EcoRI is a restriction enzyme that cleaves DNA double helices into fragments at specific sites. It is also a part of the restriction modification system.
| EcoRI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | EcoRI | ||||||||
| Pfam | PF02963 | ||||||||
| InterPro | IPR004221 | ||||||||
| SCOPe | 1na6 / SUPFAM | ||||||||
| CDD | 79lll | ||||||||
| |||||||||
In molecular biology it is used as a restriction enzyme. EcoRI creates 4 nucleotide sticky ends with 5' end overhangs of AATT. The nucleic acid recognition sequence where the enzyme cuts is G/AATTC, which has a palindromic, complementary sequence of CTTAA/G. The / in the sequence indicates which phosphodiester bond the enzyme will break in the DNA molecule. Other restriction enzymes, depending on their cut sites, can also leave 3' overhangs or blunt ends with no overhangs.
Structure
Primary structure
EcoRI contains the PD..D/EXK motif within its active site like many restriction endonucleases.
Tertiary and quaternary structure
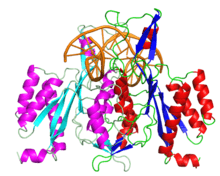
The enzyme is a homodimer of a 31 kilodalton subunit consisting of one globular domain of the α/β architecture. Each subunit contains a loop which sticks out from the globular domain and wraps around the DNA when bound.[1][2]

EcoRI has been cocrystallized with the sequence it normally cuts. This crystal was used to solve the structure of the complex 1QPS. The solved crystal structure shows that the subunits of the enzyme homodimer interact with the DNA symmetrically.[1] In the complex, two α-helices from each subunit come together to form a four-helix bundle.[3] On the interacting helices are residues Glu144 and Arg145, which interact together, forming a crosstalk ring that is believed to allow the enzyme's two active sites to communicate.[4]
Uses
Restriction enzymes, such as EcoRI, are used in a wide variety of molecular genetics techniques including cloning, DNA screening and deleting sections of DNA in vitro. Restriction enzymes, like EcoRI, that generate sticky ends of DNA are often used to cut DNA prior to ligation, as the sticky ends make the ligation reaction more efficient. EcoRI can exhibit non-site-specific cutting, known as star activity, depending on the conditions present in the reaction. Conditions that can induce star activity when using EcoRI include low salt concentration, high glycerol concentration, excessive amounts of enzyme present in the reaction, high pH and contamination with certain organic solvents. The cut made by the Eco RI enzyme produces sticky ends on the vector mainly plasmids or viral DNA's.[5]
References
- Pingoud A, Jeltsch A (September 2001). "Structure and function of type II restriction endonucleases". Nucleic Acids Research. 29 (18): 3705–27. doi:10.1093/nar/29.18.3705. PMC 55916. PMID 11557805.
- Kurpiewski MR, Engler LE, Wozniak LA, Kobylanska A, Koziolkiewicz M, Stec WJ, Jen-Jacobson L (October 2004). "Mechanisms of coupling between DNA recognition specificity and catalysis in EcoRI endonuclease". Structure. 12 (10): 1775–88. doi:10.1016/j.str.2004.07.016. PMID 15458627.
- Bitinaite J, Wah DA, Aggarwal AK, Schildkraut I (September 1998). "FokI dimerization is required for DNA cleavage". Proceedings of the National Academy of Sciences of the United States of America. 95 (18): 10570–5. Bibcode:1998PNAS...9510570B. doi:10.1073/pnas.95.18.10570. PMC 27935. PMID 9724744.
- Kim YC, Grable JC, Love R, Greene PJ, Rosenberg JM (September 1990). "Refinement of Eco RI endonuclease crystal structure: a revised protein chain tracing". Science. 249 (4974): 1307–9. Bibcode:1990Sci...249.1307K. doi:10.1126/science.2399465. PMID 2399465.
- "Archived copy". Archived from the original on 2012-10-15. Retrieved 2010-01-21.CS1 maint: archived copy as title (link)