Early-onset Alzheimer's disease
Early-onset Alzheimer's disease, also called early-onset Alzheimer's, younger-onset Alzheimer's [1]or early-onset AD, is Alzheimer's disease diagnosed before the age of 65. It is an uncommon form of Alzheimer's, accounting for only 5–10% of all Alzheimer's cases. About 60% have a positive family history of Alzheimer’s and 13% of them are inherited in an autosomal dominant manner. Most cases of early-onset Alzheimer's, however, share the same traits as the "late-onset" form and are not caused by genetic mutations. And little is understood about how it starts.
| Early-onset Alzheimer's disease | |
|---|---|
| Specialty | Neurology |
Nonfamilial early-onset AD can develop in people who are in their 30s or 40s, but this is extremely rare.[2] The majority of people with early-onset Alzheimer's are in their 50s or early 60s.
History of Alzheimer's disease
The symptoms of the disease as a distinct nosologic entity were first identified by Emil Kraepelin, and the characteristic neuropathology was first observed by Alois Alzheimer in 1906. In this sense, the disease was co-discovered by Kraepelin and Alzheimer, who worked in Kraepelin's laboratory. Because of the overwhelming importance Kraepelin attached to finding the neuropathological basis of psychiatric disorders, Kraepelin made the decision that the disease would bear Alzheimer's name.[3]
Familial Alzheimer's disease
Familial Alzheimer's disease (FAD) or early-onset familial Alzheimer's disease (EOFAD) is an uncommon form of Alzheimer's disease that usually strikes earlier in life, defined as before the age of 65 (usually between 30 and 60 years of age) and is inherited in an autosomal dominant fashion, identified by genetics and other characteristics such as the age of onset. Familial AD requires the patient to have at least one first-degree relative with a history of EOAD. FAD usually implies to multiple persons affected in one or more generation.[4] Nonfamilial cases of AD are referred to as "sporadic" AD, where genetic risk factors are minor or unclear.
While early-onset familial AD is estimated to account for only 1% of total Alzheimer's disease,[2] it has presented a useful model in studying various aspects of the disorder. Currently, the early-onset familial AD gene mutations guide the vast majority of animal model-based therapeutic discovery and development for AD.
Clinical features
Alzheimer's disease (AD) is the most common cause of dementia and usually occurs in old age. It is invariably fatal, generally within 10 years of the first signs. Early signs of AD include unusual memory loss, particularly in remembering recent events and the names of people and things, logopenic primary progressive aphasia. As the disease progresses, the patient exhibits more serious problems, becoming subject to mood swings and unable to perform complex activities such as driving. Other common findings include confusion, poor judgement, language disturbance, agitation, withdrawal, hallucinations, seizures, Parkinsonian features, increased muscle tone, myoclonus, incontinence, and mutism.[5] In the latter stages, they forget how to do simple things such as brushing their hair and then require full-time care.
Histologically, familial AD is practically indistinguishable from other forms of the disease. Deposits of amyloid can be seen in sections of brain tissue. This amyloid protein forms plaques and neurofibrillary tangles that progress through the brain. Very rarely, the plaque may be unique, or uncharacteristic of AD; this can happen when a mutation occurs in one of the genes that creates a functional, but malformed, protein instead of the ineffective gene products that usually result from mutations.
The underlying neurobiology of this disease is just recently starting to be understood. Researchers have been working on mapping the inflammation pathways associated with the development, progression, and degenerative properties of AD. The major molecules involved in these pathways include glial cells (specifically astrocytes and microglia), beta-amyloid, and proinflammatory compounds. As neurons are injured and die throughout the brain, connections between networks of neurons may break down, and many brain regions being to shrink. By the final stages of Alzheimer's, this process - called brain atrophy - is widespread, causing significant loss of brain volume. This loss of brain volume affects ones ability to live and function properly, ultimately being fatal. [6]
Beta-amyloid is a small piece of a larger protein called the amyloid precursor protein (APP). Once APP is activated, it is cut into smaller sections of other proteins. One of the fragments produced in this cutting process is β-amyloid. β-amyloid is “stickier” than any other fragment produced from cut-up APP, so it starts an accumulation process in the brain, which is due to various genetic and biochemical abnormalities. Eventually, the fragments form oligomers, then fibrils, beta-sheets, and finally plaques. The presence of β-amyloid plaques in the brain causes the body to recruit and activate microglial cells and astrocytes.
Genetics
Familial Alzheimer disease is caused by a mutation in one of at least three genes, which code for presenilin 1, presenilin 2, and amyloid precursor protein (APP).[7][8][9] Other gene mutations are in study.
PSEN1 – Presenilin 1
The presenilin 1 gene (PSEN1 located on chromosome 14) was identified by Sherrington (1995)[10] and multiple mutations have been identified. Mutations in this gene cause familial Alzheimer's type 3 with certainty and usually under 50 years old. This type accounts for 30-70% of EOFAD. [11]This protein has been identified as part of the enzymatic complex that cleaves amyloid beta peptide from APP (see below).
The gene contains 14 exons, and the coding portion is estimated at 60 kb, as reported by Rogaev (1997)[12] and Del-Favero (1999).[13] The protein the gene codes for (PS1) is an integral membrane protein. As stated by Ikeuchi (2002)[14] it cleaves the protein Notch1 so is thought by Koizumi (2001)[15] to have a role in somitogenesis in the embryo. It also has an action on an amyloid precursor protein, which gives its probable role in the pathogenesis of FAD. Homologs of PS1 have been found in plants, invertebrates and other vertebrates.
Some of the mutations in the gene, of which over 90 are known, include: His163Arg, Ala246Glu, Leu286Val and Cys410Tyr. Most display complete penetrance, but a common mutation is Glu318Gly and this predisposes individuals to familial AD, with a study by Taddei (2002)[16] finding an incidence of 8.7% in patients with familial AD.
PSEN2 – Presenilin 2
The presenilin 2 gene (PSEN2) is very similar in structure and function to PSEN1. It is located on chromosome 1 (1q31-q42), and mutations in this gene cause type 4 FAD. This type accounts for less than 5% of all EOFAD cases.[17] The gene was identified by Rudolph Tanzi and Jerry Schellenberg in 1995.[18] A subsequent study by Kovacs (1996)[19] showed that PS1 and PS2 proteins are expressed in similar amounts, and in the same organelles as each other, in mammalian neuronal cells. Levy-Lahad (1996)[20] determined that PSEN2 contained 12 exons, 10 of which were coding exons, and that the primary transcript encodes a 448-amino-acid polypeptide with 67% homology to PS1. This protein has been identified as part of the enzymatic complex that cleaves amyloid beta peptide from APP (see below).
The mutations have not been studied as much as PSEN1, but distinct allelic variants have been identified. These include Asn141Ile, which was identified first by Rudolph Tanzi and Jerry Schellenberg in Volga German families with familial Alzheimer disease (Levy-Lahad et al. Nature, 1995). One of these studies by Nochlin (1998) found severe amyloid angiopathy in the affected individuals in a family. This phenotype may be explained by a study by Tomita (1997)[21] suggesting that the Asn141Ile mutation alters amyloid precursor protein (APP) metabolism causing an increased rate of protein deposition into plaques.
Other allelic variants are Met239Val which was identified in an Italian pedigree by Rogaev (1995)[22] who also suggested early on that the gene may be similar to PSEN1, and an Asp439Ala mutation in exon 12 of the gene which is suggest by Lleo (2001)[23] to change the endoproteolytic processing of the PS2.
APP – amyloid beta (A4) precursor protein
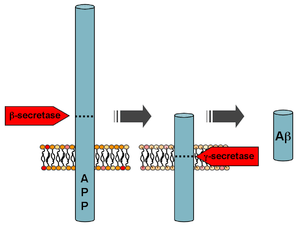
Mutations to the amyloid beta A4 precursor protein (APP) located on the long arm of chromosome 21 (21q21.3) cause familial Alzheimer disease.[9]
[24] This type accounts for no more than 10-15% of EOFAD. [25]
Two of the different APP mutations identified and characterized are the Swedish mutation[26] and the Arctic mutation.[27] Functional analyses of these mutations have significantly increased the understanding of the disease pathogenesis. Whereas the Swedish mutation located at the cleavage site for β-secretase, results in an overall higher production of Aβ peptides by increasing the β-secretory cleavage,[28] the Arctic mutation leads to a conformation change of the Aβ peptide and increased formation of toxic Aβ protofibrils.[29]
Pathophysiology
Following cleavage by β-secretase, APP is cleaved by a membrane-bound protein complex called γ-secretase to generate Aβ.[30] Presenilins 1 and 2 are the enzymatic centers of this complex along with nicastrin, Aph1, and PEN-2. Alpha-secretase cleavage of APP, which precludes the production of Aβ, is the most common processing event for APP. 21 allelic mutations have been discovered in the APP gene. These guarantee onset of early-onset familial Alzheimer disease and all occur in the region of the APP gene that encodes the Aβ domain.
Genetic testing
Genetic testing is available for symptomatic individuals and asymptomatic relatives.[8] Among families with EOFAD, 40-80% will have a detectable mutation in the APP, PSEN1, or PSEN2 gene. Therefore, some families with EOFAD will not have an identifiable mutation by current testing.
Impact of early-onset Alzheimer's
The atypical lifecourse timing of early-onset Alzheimer's means that it presents distinctive impacts upon experience. For example, the disease can have devastating effects on the careers, caretakers and family members of patients.[31][32]
Those who are working lose their ability to perform their jobs competently, and are forced into early retirement. When this can be predicted, employees must discuss their future with their employers and the loss of skills they expect to face.[33] Those who are forced to retire early may not have access to the full range of benefits available to those who retire at the minimum age set by the government.[33] With some jobs, a mistake may have devastating consequences on a large number of people, and cases have been reported in which a person with early-onset Alzheimer's who is unaware of their condition has caused distress.[34]
Younger people with Alzheimer's may also lose their ability to take care of their own needs, such as money management.[35]
It has also been highlighted, however, that conceptualizations of Alzheimer's and ageing should resist the notion that there are two distinct conditions.[36] A binary model, which focuses in particular on the needs of younger people, could lead to the challenges experienced by older people being understated.[37]
See also
- Still Alice (novel) and the movie Still Alice, whose main protagonist has EOAD
- Spirit Unforgettable, a documentary film about the farewell tour of musician John Mann and his band Spirit of the West following his diagnosis with early-onset Alzheimer's
- Thanmathra (film), an award-winning Indian film detailing the effects of early-onset Alzheimer's disease on a father and his relationship with his son.
References
- "Younger/ Early-onset Alzheimer's". Alzheimer's Association. Retrieved 9 July 2020.
- Harvey RJ, Skelton-Robinson M, Rossor MN (September 2003). "The prevalence and causes of dementia in people under the age of 65 years". Journal of Neurology, Neurosurgery, and Psychiatry. 74 (9): 1206–9. doi:10.1136/jnnp.74.9.1206. PMC 1738690. PMID 12933919.
- Weber MM (1997). "Aloys Alzheimer, a coworker of Emil Kraepelin". Journal of Psychiatric Research. 31 (6): 635–43. doi:10.1016/S0022-3956(97)00035-6. PMID 9447568.
- Bird, Thomas D. (1993), Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Early-Onset Familial Alzheimer Disease – ARCHIVED CHAPTER, FOR HISTORICAL REFERENCE ONLY", GeneReviews®, University of Washington, Seattle, PMID 20301414, retrieved 2020-05-07
- Bird, Thomas D. (1993), Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Early-Onset Familial Alzheimer Disease – ARCHIVED CHAPTER, FOR HISTORICAL REFERENCE ONLY", GeneReviews®, University of Washington, Seattle, PMID 20301414, retrieved 2020-05-07
- "What Happens to the Brain in Alzheimer's Disease?". National Institute on Aging. Retrieved 2020-05-07.
- Bertram L, Tanzi RE (October 2008). "Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses". Nature Reviews. Neuroscience. 9 (10): 768–78. doi:10.1038/nrn2494. PMID 18802446. S2CID 5946769.
- Williamson J, Goldman J, Marder KS (March 2009). "Genetic aspects of Alzheimer disease". The Neurologist. 15 (2): 80–6. doi:10.1097/NRL.0b013e318187e76b. PMC 3052768. PMID 19276785.
- Ertekin-Taner N (August 2007). "Genetics of Alzheimer's disease: a centennial review". Neurologic Clinics. 25 (3): 611–67, v. doi:10.1016/j.ncl.2007.03.009. PMC 2735049. PMID 17659183.
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. (June 1995). "Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease". Nature. 375 (6534): 754–60. Bibcode:1995Natur.375..754S. doi:10.1038/375754a0. PMID 7596406. S2CID 4308372.
- Bird, Thomas D. (1993), Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Early-Onset Familial Alzheimer Disease – ARCHIVED CHAPTER, FOR HISTORICAL REFERENCE ONLY", GeneReviews®, University of Washington, Seattle, PMID 20301414, retrieved 2020-05-07
- Rogaev EI, Sherrington R, Wu C, Levesque G, Liang Y, Rogaeva EA, et al. (March 1997). "Analysis of the 5' sequence, genomic structure, and alternative splicing of the presenilin-1 gene (PSEN1) associated with early onset Alzheimer disease". Genomics. 40 (3): 415–24. doi:10.1006/geno.1996.4523. PMID 9073509.
- Del-Favero J, Goossens D, Van den Bossche D, Van Broeckhoven C (March 1999). "YAC fragmentation with repetitive and single-copy sequences: detailed physical mapping of the presenilin 1 gene on chromosome 14". Gene. 229 (1–2): 193–201. doi:10.1016/S0378-1119(99)00023-2. PMID 10095119.
- Ikeuchi T, Sisodia SS (2002). "Cell-free generation of the notch1 intracellular domain (NICD) and APP-CTfgamma: evidence for distinct intramembranous "gamma-secretase" activities". Neuromolecular Medicine. 1 (1): 43–54. doi:10.1385/NMM:1:1:43. PMID 12025815. S2CID 21552663.
- Koizumi K, Nakajima M, Yuasa S, Saga Y, Sakai T, Kuriyama T, et al. (April 2001). "The role of presenilin 1 during somite segmentation". Development. 128 (8): 1391–402. PMID 11262239.
- Taddei K, Fisher C, Laws SM, Martins G, Paton A, Clarnette RM, et al. (2002). "Association between presenilin-1 Glu318Gly mutation and familial Alzheimer's disease in the Australian population". Molecular Psychiatry. 7 (7): 776–81. doi:10.1038/sj.mp.4001072. PMID 12192622.
- Bird, Thomas D. (1993), Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Early-Onset Familial Alzheimer Disease – ARCHIVED CHAPTER, FOR HISTORICAL REFERENCE ONLY", GeneReviews®, University of Washington, Seattle, PMID 20301414, retrieved 2020-05-07
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, et al. (August 1995). "Candidate gene for the chromosome 1 familial Alzheimer's disease locus". Science. 269 (5226): 973–7. Bibcode:1995Sci...269..973L. doi:10.1126/science.7638622. PMID 7638622.
- Kovacs DM, Fausett HJ, Page KJ, Kim TW, Moir RD, Merriam DE, et al. (February 1996). "Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells". Nature Medicine. 2 (2): 224–9. doi:10.1038/nm0296-224. PMID 8574969. S2CID 25596140.
- Levy-Lahad E, Poorkaj P, Wang K, Fu YH, Oshima J, Mulligan J, Schellenberg GD (June 1996). "Genomic structure and expression of STM2, the chromosome 1 familial Alzheimer disease gene". Genomics. 34 (2): 198–204. doi:10.1006/geno.1996.0266. PMID 8661049.
- Tomita T, Maruyama K, Saido TC, Kume H, Shinozaki K, Tokuhiro S, et al. (March 1997). "The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue". Proceedings of the National Academy of Sciences of the United States of America. 94 (5): 2025–30. Bibcode:1997PNAS...94.2025T. doi:10.1073/pnas.94.5.2025. JSTOR 41579. PMC 20036. PMID 9050898.
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, et al. (August 1995). "Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene". Nature. 376 (6543): 775–8. Bibcode:1995Natur.376..775R. doi:10.1038/376775a0. PMID 7651536. S2CID 4259326.
- Lleó A, Blesa R, Gendre J, Castellví M, Pastor P, Queralt R, Oliva R (November 2001). "A novel presenilin 2 gene mutation (D439A) in a patient with early-onset Alzheimer's disease". Neurology. 57 (10): 1926–8. doi:10.1212/WNL.57.10.1926. PMID 11723295.
- Malenka EJ, Nestler SE, Hyman RC (2009). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. ISBN 9780071481274.
- Bird, Thomas D. (1993), Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Early-Onset Familial Alzheimer Disease – ARCHIVED CHAPTER, FOR HISTORICAL REFERENCE ONLY", GeneReviews®, University of Washington, Seattle, PMID 20301414, retrieved 2020-05-07
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L (August 1992). "A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid". Nature Genetics. 1 (5): 345–7. doi:10.1038/ng0892-345. PMID 1302033. S2CID 20046036.
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, et al. (September 2001). "The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation" (PDF). Nature Neuroscience. 4 (9): 887–93. doi:10.1038/nn0901-887. PMID 11528419.
- Johnston JA, Cowburn RF, Norgren S, Wiehager B, Venizelos N, Winblad B, et al. (November 1994). "Increased beta-amyloid release and levels of amyloid precursor protein (APP) in fibroblast cell lines from family members with the Swedish Alzheimer's disease APP670/671 mutation". FEBS Letters. 354 (3): 274–8. doi:10.1016/0014-5793(94)01137-0. PMID 7957938.
- Johansson AS, Berglind-Dehlin F, Karlsson G, Edwards K, Gellerfors P, Lannfelt L (June 2006). "Physiochemical characterization of the Alzheimer's disease-related peptides A beta 1-42Arctic and A beta 1-42wt". The FEBS Journal. 273 (12): 2618–30. doi:10.1111/j.1742-4658.2006.05263.x. PMID 16817891.
- Chow VW, Mattson MP, Wong PC, Gleichmann M (March 2010). "An overview of APP processing enzymes and products". Neuromolecular Medicine. 12 (1): 1–12. doi:10.1007/s12017-009-8104-z. PMC 2889200. PMID 20232515.
- Mayo Clinic staff, Early-onset Alzheimer's: When symptoms begin before 65, Mayo Clinic
- Mary Brophy Marcus, Family shares journey after early Alzheimer's diagnosis, USA Today (September 2, 2008).
- Living With Early-Onset Alzheimer's Disease Archived 2007-10-19 at the Wayback Machine, Cleveland Clinic Health System
- Early Onset Alzheimer's On The Rise, CBS News (March 8, 2008).
- Kathleen Fackelmann, Who thinks of Alzheimer's in someone so young?, USA Today (June 11, 2007).
- Rahman, S. (2016). Young Onset Dementia: a label too far?, Dementia Society, July 27, 2016
- Tolhurst E (2016). "The Burgeoning Interest in Young Onset Dementia: Redressing the balance or reinforcing ageism?" (PDF). The International Journal of Ageing and Later Life. 10 (2): 9–29. doi:10.3384/ijal.1652-8670.16302.
External links
| Classification |
|---|
- Early-Onset Familial Alzheimer Disease - by Thomas D Bird, MD at GeneRevies (NIH.gov)