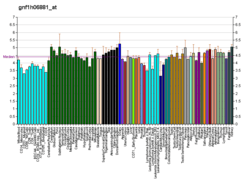
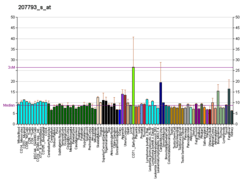
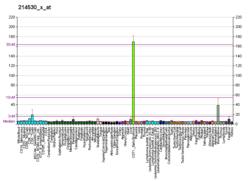
EPB41
Protein 4.1, also known as Beatty's Protein, is a protein associated with the cytoskeleton that in humans is encoded by the EPB41 gene. Protein 4.1 is a major structural element of the erythrocyte membrane skeleton. It plays a key role in regulating membrane physical properties of mechanical stability and deformability by stabilizing spectrin-actin interaction. Protein 4.1 (80 kD) interacts with spectrin and short actin filaments to form the erythrocyte membrane skeleton. Mutations of spectrin and protein 4.1 are associated with elliptocytosis or spherocytosis and anemia of varying severity.
Clinical significance
Elliptocytosis is a hematologic disorder characterized by elliptically shaped erythrocytes and a variable degree of hemolytic anemia. Inherited as an autosomal dominant, elliptocytosis results from mutation in any one of several genes encoding proteins of the red cell membrane skeleton. The form discussed here is the one found in the 1950s to be linked to Rh blood group and more recently shown to be caused by a defect in protein 4.1. 'Rh-unlinked' forms of elliptocytosis are caused by mutation in the alpha-spectrin gene (MIM 182860), the beta-spectrin gene (MIM 182870), or the band 3 gene (MIM 109270) [supplied by OMIM].[5]
Interactions
Band 4.1 has been shown to interact with:
See also
References
- GRCh38: Ensembl release 89: ENSG00000159023 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000028906 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: EPB41 erythrocyte membrane protein band 4.1 (elliptocytosis 1, RH-linked)".
- Hung LY, Tang CJ, Tang TK (October 2000). "Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex". Mol. Cell. Biol. 20 (20): 7813–25. doi:10.1128/mcb.20.20.7813-7825.2000. PMC 86375. PMID 11003675.
- Hou CL; Tang Cj; Roffler SR; Tang TK (July 2000). "Protein 4.1R binding to eIF3-p44 suggests an interaction between the cytoskeletal network and the translation apparatus". Blood. 96 (2): 747–53. PMID 10887144.
- Mattagajasingh SN, Huang SC, Hartenstein JS, Snyder M, Marchesi VT, Benz EJ (April 1999). "A nonerythroid isoform of protein 4.1R interacts with the nuclear mitotic apparatus (NuMA) protein". J. Cell Biol. 145 (1): 29–43. doi:10.1083/jcb.145.1.29. PMC 2148212. PMID 10189366.
- Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ (September 2000). "Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton". J. Biol. Chem. 275 (39): 30573–85. doi:10.1074/jbc.M004578200. PMID 10874042.
Further reading
- Conboy JG (1993). "Structure, function, and molecular genetics of erythroid membrane skeletal protein 4.1 in normal and abnormal red blood cells". Semin. Hematol. 30 (1): 58–73. PMID 8434260.
- Calinisan V, Gravem D, Chen RP, Brittin S, Mohandas N, Lecomte MC, Gascard P (2006). "New insights into potential functions for the protein 4.1 superfamily of proteins in kidney epithelium". Front. Biosci. 11: 1646–66. doi:10.2741/1911. PMID 16368544.
- Dalla Venezia N, Gilsanz F, Alloisio N, Ducluzeau MT, Benz EJ, Delaunay J (1992). "Homozygous 4.1(-) hereditary elliptocytosis associated with a point mutation in the downstream initiation codon of protein 4.1 gene". J. Clin. Invest. 90 (5): 1713–7. doi:10.1172/JCI116044. PMC 443228. PMID 1430200.
- Jöns T, Drenckhahn D (1992). "Identification of the binding interface involved in linkage of cytoskeletal protein 4.1 to the erythrocyte anion exchanger". EMBO J. 11 (8): 2863–7. doi:10.1002/j.1460-2075.1992.tb05354.x. PMC 556766. PMID 1639060.
- Subrahmanyam G, Bertics PJ, Anderson RA (1991). "Phosphorylation of protein 4.1 on tyrosine-418 modulates its function in vitro". Proc. Natl. Acad. Sci. U.S.A. 88 (12): 5222–6. doi:10.1073/pnas.88.12.5222. PMC 51844. PMID 1647028.
- Conboy JG, Chan JY, Chasis JA, Kan YW, Mohandas N (1991). "Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1". J. Biol. Chem. 266 (13): 8273–80. PMID 2022644.
- Horne WC, Prinz WC, Tang EK (1990). "Identification of two cAMP-dependent phosphorylation sites on erythrocyte protein 4.1". Biochim. Biophys. Acta. 1055 (1): 87–92. doi:10.1016/0167-4889(90)90095-U. PMID 2171679.
- Conboy J, Marchesi S, Kim R, Agre P, Kan YW, Mohandas N (1990). "Molecular analysis of insertion/deletion mutations in protein 4.1 in elliptocytosis. II. Determination of molecular genetic origins of rearrangements". J. Clin. Invest. 86 (2): 524–30. doi:10.1172/JCI114739. PMC 296755. PMID 2384598.
- Inaba M, Maede Y (1989). "O-N-acetyl-D-glucosamine moiety on discrete peptide of multiple protein 4.1 isoforms regulated by alternative pathways". J. Biol. Chem. 264 (30): 18149–55. PMID 2808371.
- Korsgren C, Cohen CM (1988). "Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3". J. Biol. Chem. 263 (21): 10212–8. PMID 2968981.
- Conboy JG, Chan J, Mohandas N, Kan YW (1988). "Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells". Proc. Natl. Acad. Sci. U.S.A. 85 (23): 9062–5. doi:10.1073/pnas.85.23.9062. PMC 282663. PMID 3194408.
- Tang TK, Leto TL, Marchesi VT, Benz EJ (1989). Expression of specific isoforms of protein 4.1 in erythroid and non-erythroid tissues. Adv. Exp. Med. Biol. Advances in Experimental Medicine and Biology. 241. pp. 81–95. doi:10.1007/978-1-4684-5571-7_12. ISBN 978-1-4684-5573-1. PMID 3223413.
- Tang TK, Leto TL, Correas I, Alonso MA, Marchesi VT, Benz EJ (1988). "Selective expression of an erythroid-specific isoform of protein 4.1". Proc. Natl. Acad. Sci. U.S.A. 85 (11): 3713–7. doi:10.1073/pnas.85.11.3713. PMC 280288. PMID 3375238.
- Conboy J, Kan YW, Shohet SB, Mohandas N (1987). "Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton". Proc. Natl. Acad. Sci. U.S.A. 83 (24): 9512–6. doi:10.1073/pnas.83.24.9512. PMC 387170. PMID 3467321.
- Correas I, Speicher DW, Marchesi VT (1986). "Structure of the spectrin-actin binding site of erythrocyte protein 4.1". J. Biol. Chem. 261 (28): 13362–6. PMID 3531202.
- Tchernia G, Mohandas N, Shohet SB (1981). "Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability". J. Clin. Invest. 68 (2): 454–60. doi:10.1172/JCI110275. PMC 370818. PMID 6894932.
- Schischmanoff PO, Winardi R, Discher DE, Parra MK, Bicknese SE, Witkowska HE, Conboy JG, Mohandas N (1995). "Defining of the minimal domain of protein 4.1 involved in spectrin-actin binding". J. Biol. Chem. 270 (36): 21243–50. doi:10.1074/jbc.270.36.21243. PMID 7673158.
- Lue RA, Marfatia SM, Branton D, Chishti AH (1994). "Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1". Proc. Natl. Acad. Sci. U.S.A. 91 (21): 9818–22. doi:10.1073/pnas.91.21.9818. PMC 44908. PMID 7937897.
- Conboy JG, Chasis JA, Winardi R, Tchernia G, Kan YW, Mohandas N (1993). "An isoform-specific mutation in the protein 4.1 gene results in hereditary elliptocytosis and complete deficiency of protein 4.1 in erythrocytes but not in nonerythroid cells". J. Clin. Invest. 91 (1): 77–82. doi:10.1172/JCI116203. PMC 329997. PMID 8423235.
External links
- erythrocyte+membrane+band+4.1+protein at the US National Library of Medicine Medical Subject Headings (MeSH)