Butylated hydroxyanisole
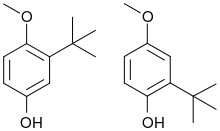
Butylated hydroxyanisole (BHA) is an antioxidant consisting of a mixture of two isomeric organic compounds, 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole. It is prepared from 4-methoxyphenol and isobutylene. It is a waxy solid used as a food additive with the E number E320. The primary use for BHA is as an antioxidant and preservative in food, food packaging, animal feed, cosmetics, rubber, and petroleum products.[3] BHA also is commonly used in medicines, such as isotretinoin, lovastatin, and simvastatin, among others.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-tert-Butyl-4-methoxyphenol and 3-tert-butyl-4-methoxyphenol (mixture) | |
| Other names
2-tert-Butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole (mixture) BOA BHA tert-Butyl-4-hydroxyanisole (1,1-Dimethylethyl)-4-methoxyphenol tert-Butyl-4-methoxyphenol Antioxyne B[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.042.315 |
| E number | E320 (antioxidants, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H16O2 | |
| Molar mass | 180.247 g·mol−1 |
| Appearance | Waxy solid |
| Density | 1.0587 g/cm3 at 20 °C |
| Melting point | 48 to 55 °C (118 to 131 °F; 321 to 328 K) |
| Boiling point | 264 to 270 °C (507 to 518 °F; 537 to 543 K) |
| Insoluble in water | |
| Solubility | Freely soluble in ethanol, methanol, propylene glycol; soluble in fats and oils |
Refractive index (nD) |
1.5303 at 589.3nm [2] |
| Related compounds | |
Related compounds |
Butylated hydroxytoluene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Antioxidant properties
Since 1947, BHA has been added to edible fats and fat-containing foods for its antioxidant properties as it prevents rancidification of food which creates objectionable odors.[4] Like butylated hydroxytoluene (BHT), the conjugated aromatic ring of BHA is able to stabilize free radicals, sequestering them. By acting as free radical scavengers, further free radical reactions are prevented.
Research
The U.S. National Institutes of Health report that BHA is reasonably anticipated to be a human carcinogen based on evidence of carcinogenicity in experimental animals. In particular, when administered in high doses as part of their diet, BHA causes papillomas and squamous cell carcinomas of the forestomach in rats and Syrian golden hamsters.[5] In mice, there is no carcinogenic effect;[5] in fact, there is evidence of a protective effect against the carcinogenicity of other chemicals.[4]
When examining human population statistics, the usual low intake levels of BHA show no significant association with an increased risk of cancer.[6] The state of California has, however, listed BHA as a carcinogen.[7]
See also
References
- "BHA and BHT". Retrieved Nov 20, 2009.
- "SciFinder — Experimental properties for 121-00-6". Retrieved Nov 20, 2009.
- Hazardous Substances Database, National Library of Medicine
- Lam, L. K.; R. P. Pai & L. W. Wattenberg (1979). "Synthesis and chemical carcinogen inhibitory activity of 2-tert-butyl-4-hydroxyanisole". J Med Chem. 22 (5): 569–71. doi:10.1021/jm00191a020. PMID 458807.
- Butylated Hydroxyanisole (BHA), CAS No. 25013-16-5 Archived 2010-06-05 at the Wayback Machine, Report on Carcinogens, Eleventh Edition, National Institutes of Health
- Botterweck AAM, Vergaen H, GoldBohm RA, KleinJans J, van den Brant PA (2007). "Intake of Butylated Hydroxyanisole and Butylated Hydroxytoluene and Stomach Cancer Risk: Results from Analyses in the Netherlands Cohort Study". Food and Chemical Toxicology. 38 (7): 599–605. doi:10.1016/S0278-6915(00)00042-9. PMID 10942321.
- "Known Carcinogens and Reproductive Toxicants (California Proposition 65)". Scorecard. Retrieved 2011-05-29.