Ethylparaben
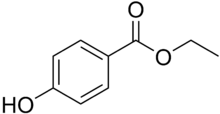
Ethylparaben (ethyl para-hydroxybenzoate) is the ethyl ester of p-hydroxybenzoic acid. Its formula is HO-C6H4-CO-O-CH2CH3. It is a member of the class of compounds known as parabens.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ethyl 4-hydroxybenzoate | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.000 |
| E number | E214 (preservatives) |
| KEGG | |
| MeSH | ethyl-p-hydroxybenzoate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O3 | |
| Molar mass | 166.176 g·mol−1 |
| Melting point | 115 to 118 °C (239 to 244 °F; 388 to 391 K) |
| Boiling point | 297 to 298 °C (567 to 568 °F; 570 to 571 K) |
| Pharmacology | |
| D01AE10 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 248 °C (478 °F; 521 K) |
| Related compounds | |
Related compounds |
Paraben Butylparaben Methylparaben Propylparaben |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is used as an antifungal preservative. As a food additive, it has E number E214.
Sodium ethyl para-hydroxybenzoate, the sodium salt of ethylparaben, has the same uses and is given the E number E215.
References
- Ethyl paraben, thegoodscentscompany.com
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
