Dock9
Dock9 (Dedicator of cytokinesis 9), also known as Zizimin1, is a large (~230 kDa) protein involved in intracellular signalling networks.[5] It is a member of the DOCK-D subfamily of the DOCK family of guanine nucleotide exchange factors that function as activators of small G proteins. Dock9 activates the small G protein Cdc42.
Discovery
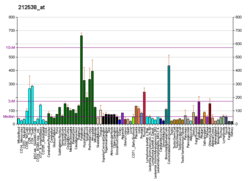
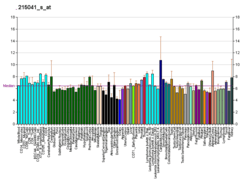
Dock9 was discovered using an affinity proteomic approach designed to identify novel activators of the small G protein Cdc42 in fibroblasts.[6] Subsequent northern blot analysis revealed that Dock9 is expressed primarily in the brain, heart, skeletal muscle, kidney, placenta and lung. Lower levels were detected in the colon, thymus, liver, small intestine and in leukocytes from peripheral blood.
Structure and Function
Dock9 shares a similar structure of two core domains (known as DHR1 and DHR2), which are shared by all DOCK family members. The C-terminal DHR2 domain functions as an atypical GEF domain for small G proteins (see Dock180: structure and function) and the DHR1 domain is known, in some DOCK-A/B/C subfamily proteins, to be involved in their recruitment to the plasma membrane. Unlike DOCK-A/B/C proteins DOCK-D proteins (including Dock9) contain an N-terminal pleckstrin homology (PH) domain that mediates their recruitment to the membrane.[7] Dock9, along with other DOCK-C/D subfamily members, can activate Cdc42 in vitro and in vivo via its DHR2 domain.[6] However, Dock9 adopts an autoinhibitory conformation that masks the DHR2 domain in its resting state.[7] The mechanism by which this autoinhibition is overcome is still unclear although in some other DOCK proteins, which also undergo autoinhibition, it requires an interaction with adaptor proteins such as ELMO.[8][9] Dock9 has also been reported to dimerise, under resting conditions, via its DHR2 domains and this study suggests that other DOCK family proteins may also behave in the same way.[10] Recent analysis of a chromosomal region associated with susceptibility to bipolar disorder revealed that single nucleotide polymorphisms in the DOCK9 gene contribute to the risk and severity of this condition.[11]
References
- GRCh38: Ensembl release 89: ENSG00000088387 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000025558 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: DOCK9 dedicator of cytokinesis 9".
- Meller N, Irani-Tehrani M, Kiosses WB, et al. (September 2002). "Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins". Nat. Cell Biol. 4 (9): 639–47. doi:10.1038/ncb835. PMID 12172552.
- Meller N, Westbrook MJ, Shannon JD, et al. (January 2008). "Function of the N-terminus of zizimin1: autoinhibition and membrane targeting". Biochem. J. 409 (2): 525–33. doi:10.1042/BJ20071263. PMC 2740492. PMID 17935486.
- Lu M, Kinchen JM, Rossman KL, et al. (August 2004). "PH domain of ELMO functions in trans to regulate Rac activation via Dock180". Nat. Struct. Mol. Biol. 11 (8): 756–62. doi:10.1038/nsmb800. PMID 15247908.
- Lu M, Kinchen JM, Rossman KL, et al. (February 2005). "A Steric-inhibition model for regulation of nucleotide exchange via the Dock180 family of GEFs". Curr. Biol. 15 (4): 371–77. doi:10.1016/j.cub.2005.01.050. PMID 15723800.
- Meller N, Irani-Tehrani M, Ratnikov BI, et al. (September 2004). "The novel Cdc42 guanine nucleotide exchange factor, zizimin1, dimerizes via the Cdc42-binding CZH2 domain". J. Biol. Chem. 279 (36): 37470–76. doi:10.1074/jbc.M404535200. PMID 15247287.
- Detera-Wadleigh SD, Liu CY, Maheshwari M, et al. (October 2007). "Sequence variation in DOCK9 and heterogeneity in bipolar disorder". Psychiatr. Genet. 17 (5): 274–86. doi:10.1097/YPG.0b013e328133f352. PMID 17728666.
Further reading
- Nakajima D, Okazaki N, Yamakawa H, et al. (2003). "Construction of expression-ready cDNA clones for KIAA genes: manual curation of 330 KIAA cDNA clones". DNA Res. 9 (3): 99–106. doi:10.1093/dnares/9.3.99. PMID 12168954.
- Kwofie MA, Skowronski J (2008). "Specific recognition of Rac2 and Cdc42 by DOCK2 and DOCK9 guanine nucleotide exchange factors". J. Biol. Chem. 283 (6): 3088–96. doi:10.1074/jbc.M705170200. PMID 18056264.
- Meller N, Westbrook MJ, Shannon JD, et al. (2008). "Function of the N-terminus of zizimin1: autoinhibition and membrane targeting". Biochem. J. 409 (2): 525–33. doi:10.1042/BJ20071263. PMC 2740492. PMID 17935486.
- Detera-Wadleigh SD, Liu CY, Maheshwari M, et al. (2007). "Sequence variation in DOCK9 and heterogeneity in bipolar disorder". Psychiatr. Genet. 17 (5): 274–86. doi:10.1097/YPG.0b013e328133f352. PMID 17728666.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Dunham A, Matthews LH, Burton J, et al. (2004). "The DNA sequence and analysis of human chromosome 13". Nature. 428 (6982): 522–8. doi:10.1038/nature02379. PMC 2665288. PMID 15057823.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Côté JF, Vuori K (2003). "Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity". J. Cell Sci. 115 (Pt 24): 4901–13. doi:10.1242/jcs.00219. PMID 12432077.
- Meller N, Irani-Tehrani M, Kiosses WB, et al. (2002). "Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins". Nat. Cell Biol. 4 (9): 639–47. doi:10.1038/ncb835. PMID 12172552.
- Kikuno R, Nagase T, Ishikawa K, et al. (1999). "Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Res. 6 (3): 197–205. doi:10.1093/dnares/6.3.197. PMID 10470851.