Disulfur difluoride
Disulfur difluoride is a halide of sulfur, with the chemical formula S2F2.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
fluorosulfanyl thiohypofluorite | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| S2F2 | |||
| Molar mass | 102.127 g/mol | ||
| Related compounds | |||
Related compounds |
O 2F 2 S 2Cl 2 SF 2 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
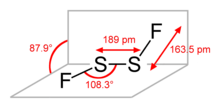
Structure
Disulfur difluoride will undergo intramolecular rearrangement in the presence of alkali elements' fluorides, yielding the isomer S=SF2:[1]
Synthesis
Silver(II) fluoride can fluorinate sulfur in a strictly dry container, and the reaction produces FS-SF:[2]
S=SF2 can be synthesized with the reaction of potassium fluorosulfite and disulfur dichloride:
Reactions
- Decomposing to sulfur tetrafluoride and sulfur when heated:
- Treated with water:
- Reacting with sulfuric acid:
- Reacting with sodium hydroxide:
- Reacting with oxygen at high pressure, using nitrogen dioxide as the catalyst:
- Condensing with sulfur difluoride at low temperatures to yield 1,3-Difluoro-trisulfane-1,1-difluoride.
- SSF2 + SF2 → FSSSF3[7]
gollark: ++remind "18:03 tomorrow" laugh at <@398575402865393665> guesses
gollark: ++remind "18:04 tomorrow" laugh at own guesses
gollark: ++remind "18:05 tomorrow" laugh at lyricly guesses
gollark: Yes you do. They just aren't very good.
gollark: 2023, after the introduction of MinoteaurScript™ and also 2022 if you count the Turing-complete recursive templating system.
References
- Davis, R.Wellington (1986). "The microwave spectrum of the pyramidal isomer of disulfur difluoride: S=SF2". Journal of Molecular Spectroscopy. 116 (2): 371–383. doi:10.1016/0022-2852(86)90134-7.
- Davis, R.Wellington; Firth, Steven (1991). "The microwave spectrum of the chain isomer of disulfur difluoride: FS-SF". Journal of Molecular Spectroscopy. 145 (2): 225. doi:10.1016/0022-2852(91)90109-N.
- 张青莲 (1991). 《无机化学丛书》第五卷:氧、硫、硒分族 (in Chinese). Beijing: Science Press. p. 179. ISBN 978-7-03-002238-7.
- Справочник химика / Редкол.: Никольский Б.П. и др.. — 3-е изд., испр. — Л.: Химия, 1971. — Т. 2. — 1168 с. (in Russian)
- Химическая энциклопедия / Редкол.: Кнунянц И.Л. и др.. — М.: Советская энциклопедия, 1995. — Т. 4. — 639 с. — ISBN 978-5-85270-092-6 (in Russian)
- Лидин Р.А. и др. Химические свойства неорганических веществ: Учеб. пособие для вузов. — 3-е изд., испр. — М.: Химия, 2000. — 480 с. — ISBN 978-5-7245-1163-6 (in Russian)
- Lösking, O.; Willner, H.; Baumgärtel, H.; Jochims, H. W.; Rühl, E. (November 1985). "Chalkogenfluoride in niedrigen Oxydationsstufen. X Thermochemische Daten und Photoionisations-Massenspektren von SSF2, FSSF, SF3SF und SF3SSF". Zeitschrift für anorganische und allgemeine Chemie. 530 (11): 169–177. doi:10.1002/zaac.19855301120.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.