Diethylamine
Diethylamine is an organic compound with the formula (CH3CH2)2NH. It is a secondary amine. It is a flammable, weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear brown due to impurities. It has a strong ammonia-like odor.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N-Ethylethanamine | |
| Other names
(Diethyl)amine Diethylamine (deprecated[2]) | |
| Identifiers | |
3D model (JSmol) |
|
| 605268 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.380 |
| EC Number |
|
| MeSH | diethylamine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1154 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H11N | |
| Molar mass | 73.139 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | fishy, ammonical |
| Density | 0.7074 g mL−1 |
| Melting point | −49.80 °C; −57.64 °F; 223.35 K |
| Boiling point | 54.8 to 56.4 °C; 130.5 to 133.4 °F; 327.9 to 329.5 K |
| Miscible | |
| log P | 0.657 |
| Vapor pressure | 24.2–97.5 kPa |
Henry's law constant (kH) |
150 μmol Pa−1 kg−1 |
| Acidity (pKa) | 10.98 (of ammonium form) |
| -56.8·10−6 cm3/mol | |
Refractive index (nD) |
1.385 |
| Thermochemistry | |
Heat capacity (C) |
178.1 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−131 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−3.035 MJ mol−1 |
| Hazards | |
| Safety data sheet | hazard.com |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H225, H302, H312, H314, H332 |
| P210, P280, P305+351+338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | −23 °C (−9 °F; 250 K) |
| 312 °C (594 °F; 585 K) | |
| Explosive limits | 1.8–10.1% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
540 mg/kg (rat, oral) 500 mg/kg (mouse, oral)[3] |
LC50 (median concentration) |
4000 ppm (rat, 4 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 25 ppm (75 mg/m3)[4] |
REL (Recommended) |
TWA 10 ppm (30 mg/m3) ST 25 ppm (75 mg/m3)[4] |
IDLH (Immediate danger) |
200 ppm[4] |
| Related compounds | |
Related amines |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production and uses
Diethylamine is made by the alumina-catalyzed reaction of ethanol and ammonia. It is obtained together with ethylamine and triethylamine. Annual production of three ethylamines was estimated in 2000 to be 80,000,000 kg.[5]
It is used in the production of corrosion inhibitor N,N-diethylaminoethanol, by reaction with ethylene oxide. It is also a precursor to a wide variety of other commercial products.
Diethylamine is also sometimes used in the illicit production of LSD[6].
Supramolecular structure
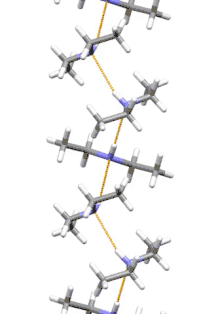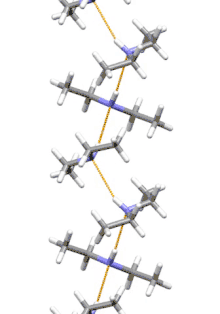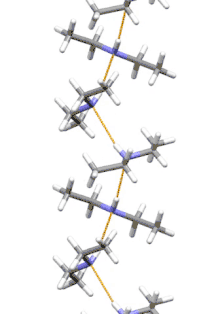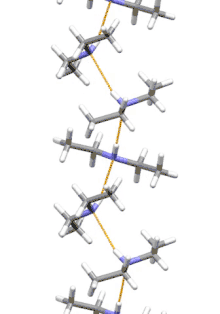
Diethylamine is the smallest and simplest molecule that features a supramolecular helix as its lowest energy aggregate. Other similarly sized hydrogen-bonding molecules favor cyclic structures.[7]
Safety
Diethylamine has low toxicity, but the vapor causes transient impairment of vision.[5]
References
- Merck Index, 12th Edition, 3160
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 671. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- "Diethylamine". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0209". National Institute for Occupational Safety and Health (NIOSH).
- Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.CS1 maint: multiple names: authors list (link)
- Shulgin, Alexander. "Erowid Online Books:"TIHKAL" - #26 LSD-25". www.erowid.org. Retrieved 12 August 2019.
- Felix Hanke; Chloe J. Pugh; Ellis F. Kay; Joshua B. Taylor; Stephen M. Todd; Craig M. Robertson; Benjamin J. Slater; Alexander Steiner (2018). "The simplest supramolecular helix". Chemical Communications. 54. doi:10.1039/C8CC03295E.
