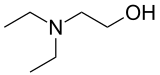
Diethylethanolamine
Diethylethanolamine (DEAE) is a chemical compound with the molecular formula C6H15NO. It is used as a precursor in the production of a variety of chemical commodities such as the local anesthetic procaine. It can be reacted with 4-aminobenzoic acid to make procaine. DEAE can be used as a precursor for DEAE-cellulose resin, which is commonly used in ion exchange chromatography. DEAE can also be conveniently obtained from renewable sources. It is chemically stable and able to absorb carbon dioxide (CO2) from its surroundings. In solution, it can decrease the surface tension of water when the temperature is increased.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(Diethylamino)ethan-1-ol | |
| Other names
2-(Diethylamino)ethanol Diethylaminoethanol 2-Diethylaminoethanol N,N-Diethyl-2-aminoethanol N,N-Diethylethanolamine Diethyl(2-hydroxyethyl)amine (2-Hydroxyethyl)diethylamine 2-Diethylaminoethyl alcohol 2-Hydroxytriethylamine | |
| Identifiers | |
3D model (JSmol) |
|
| 741863 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.587 |
| EC Number |
|
| MeSH | 2-diethylaminoethanol |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2686 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H15NO | |
| Molar mass | 117.192 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Ammoniacal |
| Density | 884 mg mL−1 |
| Melting point | −70 °C; −94 °F; 203 K [1] |
| Boiling point | 161.1 °C; 321.9 °F; 434.2 K |
| miscible[1] | |
| log P | 0.769 |
| Vapor pressure | 100 Pa (at 20 °C) |
Refractive index (nD) |
1.441–1.442 |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H226, H302, H312, H314, H317, H332 |
| P280, P305+351+338, P310 | |
| Flash point | 50 °C (122 °F; 323 K) |
| Explosive limits | 1.4–11.7% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
LC50 (median concentration) |
924 ppm (rat, 4 hr) 1027 ppm (mouse)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 10 ppm (50 mg/m3) [skin][1] |
REL (Recommended) |
TWA 10 ppm (50 mg/m3) [skin][1] |
IDLH (Immediate danger) |
100 ppm[1] |
| Related compounds | |
Related alkanols |
|
Related compounds |
Diethylhydroxylamine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
Diethylethanolamine is used as a corrosion inhibitor in steam and condensate lines by neutralizing carbonic acid and scavenging oxygen.
Preparation
Diethylethanolamine is prepared commercially by the reaction of diethylamine and ethylene oxide.[4]
- (C2H5)2NH + cyclo(CH2CH2)O → (C2H5)2NCH2CH2OH
It is also possible to prepare it by the reaction of diethylamine and ethylene chlorohydrin.[5]
Safety
Diethylethanolamine is an irritant to the eyes, skin, and respiratory system. The Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have set occupational exposure limits for workers handling the chemical at 10 ppm (50 mg/m3) over an eight-hour workday.[6]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0210". National Institute for Occupational Safety and Health (NIOSH).
- "2-Diethylaminoethanol". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Fu, Dong; Wang, LeMeng; Tian, XiangFeng. "Experiments and model for the surface tension of DEAE-PZ and DEAE-MEA aqueous solutions". The Journal of Chemical Thermodynamics. 105: 71–75. doi:10.1016/j.jct.2016.10.007.
- Bollmeier, Jr., Allen F. (1999). "Alkanolamines". In Kroschwitz, Jacqueline I. (ed.). Kirk-Othmer Encylclopedia of Chemical Technology. 2 (4th ed.). New York: John Wiley & Sons, Inc. pp. 1–34. ISBN 978-0471419617.
- "Diethylaminoethanol". USDA. 2001-02-15. Retrieved 2012-08-28.
- "NIOSH Pocket Guide to Chemical Hazards". CDC. 2011-04-04. Retrieved 2013-11-08.