Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene (or their equivalents).[1] An alkyl group is a piece of a molecule with the general formula CnH2n+1, where n is the integer depicting the number of carbons linked together. For example, a methyl group (n = 1, CH3) is a fragment of a methane molecule (CH4). Alkylating agents use selective alkylation by adding the desired aliphatic carbon chain to the previously chosen starting molecule. This is one of many known chemical syntheses. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character.
In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline.[2]
In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.

Nucleophilic alkylating agents
Nucleophilic alkylating agents deliver the equivalent of an alkyl anion (carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent bond between the alkyl group and the electrophile. The counterion, which is a cation such as lithium, can be removed and washed away in the work-up. Examples include the use of organometallic compounds such as Grignard (organomagnesium), organolithium, organocopper, and organosodium reagents. These compounds typically can add to an electron-deficient carbon atom such as at a carbonyl group. Nucleophilic alkylating agents can displace halide substituents on a carbon atom through the SN2 mechanism. With a catalyst, they also alkylate alkyl and aryl halides, as exemplified by Suzuki couplings.
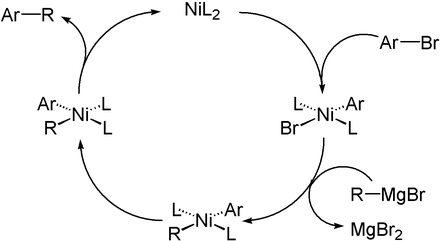
The SN2 mechanism is not available for aryl substituents, where the trajectory to attack the carbon atom would be inside the ring. Thus only reactions catalyzed by organometallic catalysts are possible.
Electrophilic alkylating agents
C-alkylation
C-alkylation is a process for the formation of carbon-carbon bonds. For alkylation at carbon, the electrophilicity of alkyl halides is enhanced by the presence of a Lewis acid such as aluminium trichloride. Lewis acids are particularly suited for C-alkylation. C-alkylation can also be effected by alkenes in the presence of acids.
N-and P-alkylation
N- and P-alkylation are important processes for the formation of carbon-nitrogen and carbon-phosphorus bonds.
Amines are readily alkylated. The rate of alkylation follows the order tertiary amine < secondary amine < primary amine. Typical alkylating agents are alkyl halides. Industry often relies on green chemistry methods involving alkylation of amines with alcohols, the byproduct being water. Hydroamination is another green method for N-alkylation.
In the Menshutkin reaction, a tertiary amine is converted into a quaternary ammonium salt by reaction with an alkyl halide. Similar reactions occur when tertiary phosphines are treated with alkyl halides, the products being phosphonium salts.
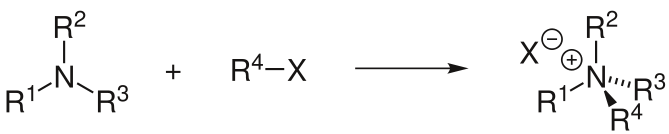
S-alkylation
Thiols are readily alkylated to give thioethers.[3] The reaction is typically conducted in the presence of a base or using the conjugate base of the thiol. Thioethers undergo alkylation to give sulfonium ions.
O-alkylation
Alcohols alkylate to give ethers:
- ROH + R'X → ROR'
When the alkylating agent is an alkyl halide, the conversion is called the Williamson ether synthesis. Alcohols are also good alkylating agents in the presence of suitable acid catalysts. For example, most methyl amines are prepared by alkylation of ammonia with methanol. The alkylation of phenols is particularly straightforward since it is subject to fewer competing reactions.[4]
- (with Na+ as a spectator ion)
More complex alkylation of a alcohols and phenols involve ethoxylation. Ethylene oxide is the alkylating group in this reaction.
Oxidative addition to metals
In the process called oxidative addition, low-valent metals often react with alkylating agents to give metal alkyls. This reaction is one step in the Cativa process for the synthesis of acetic acid from methyl iodide. Many cross coupling reactions proceed via oxidative addition as well.
Electrophilic alkylating agents
Electrophilic alkylating agents deliver the equivalent of an alkyl cation. Alkyl halides are typical alkylating agents. Trimethyloxonium tetrafluoroborate and triethyloxonium tetrafluoroborate are particularly strong electrophiles due to their overt positive charge and an inert leaving group (dimethyl or diethyl ether). Dimethyl sulfate is intermediate in electrophilicity.

Hazards
Electrophilic, soluble alkylating agents are often toxic and carcinogenic, due to their tendency to alkylate DNA. This mechanism of toxicity is relevant to the function of anti-cancer drugs in the form of alkylating antineoplastic agents. Some chemical weapons such as mustard gas function as alkylating agents. Alkylated DNA either does not coil or uncoil properly, or cannot be processed by information-decoding enzymes.
Catalysts

Electrophilic alkylations use Lewis acids and Brønsted acids, sometimes both. Classically, Lewis acids, e.g., aluminium trichloride, are employed when the alkyl halide are used. Brønsted acids are used when alkylating with olefins. Typical catalysts are zeolites, i.e. solid acid catalysts, and sulfuric acid. Silicotungstic acid is used to manufacture ethyl acetate by the alkylation of acetic acid by ethylene:[6]
- C2H4 + CH3CO2H → CH3CO2C2H5
In biology
Methylation is the most common type of alkylation. Methylation in nature is often effected by vitamin B12- and radical-SAM-based enzymes.
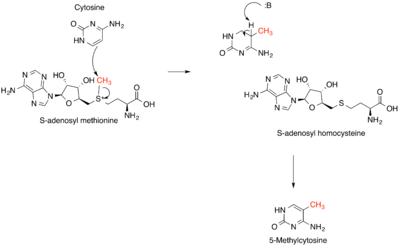
In methanogenesis, coenzyme M is methylated by tetrahydromethanopterin.
Commodity chemicals
Several commodity chemicals are produced by alkylation. Included are several fundamental benzene-based feedstocks such as ethylbenzene (precursor to styrene), cumene (precursor to phenol and acetone), linear alkylbenzene sulfonates (for detergents).[7]
Oil refining

In a conventional oil refinery, isobutane is alkylated with low-molecular-weight alkenes (primarily a mixture of propene and butene) in the presence of a Brønsted acid catalyst, which can include solid acids (zeolites). The catalyst protonates the alkenes (propene, butene) to produce carbocations, which alkylate isobutane. The product, called "alkylate", is composed of a mixture of high-octane, branched-chain paraffinic hydrocarbons (mostly isoheptane and isooctane). Alkylate is a premium gasoline blending stock because it has exceptional antiknock properties and is clean burning. Alkylate is also a key component of avgas. By combining fluid catalytic cracking, polymerization, and alkylation refineries can obtain a gasoline yield of 70 percent. The widespread use of sulfuric acid and hydrofluoric acid in refineries poses significant environmental risks.[8]
See also
- Hydrodealkylation
- Transalkylation
- Alkynylation
- Friedel–Crafts reaction
- Category:Alkylating agents
- Category:Ethylating agents
- Category:Methylating agents
References
- March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. ISBN 0-471-85472-7
- Stefanidakis, G.; Gwyn, J.E. (1993). "Alkylation". In John J. McKetta (ed.). Chemical Processing Handbook. CRC Press. pp. 80–138. ISBN 0-8247-8701-3.
- D. Landini And F. Rolla (1978). "Sulfide Synthesis In Preparation Of Dialkyl And Alkyl Aryl Sulfides: Neopentyl Phenyl Sulfide". Org. Synth. 58: 143. doi:10.15227/orgsyn.058.0143.CS1 maint: uses authors parameter (link)
- G. S. Hiers and F. D. Hager (1941). "Anisole". Organic Syntheses.; Collective Volume, 1, p. 58
- H. Perst, D. G. Seapy (2008). "Triethyloxonium Tetrafluoroborate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt223.pub2.CS1 maint: uses authors parameter (link)
- Misono, Makoto (2009). "Recent progress in the practical applications of heteropolyacid and perovskite catalysts: Catalytic technology for the sustainable society". Catalysis Today. 144 (3–4): 285–291. doi:10.1016/j.cattod.2008.10.054.
- Bipin V. Vora, Joseph A. Kocal, Paul T. Barger, Robert J. Schmidt, James A. Johnson (2003). "Alkylation". Kirk‐Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0112112508011313.a01.pub2.CS1 maint: uses authors parameter (link)
- Michael Röper, Eugen Gehrer, Thomas Narbeshuber, Wolfgang Siegel "Acylation and Alkylation" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000. doi:10.1002/14356007.a01_185
External links
- Macrogalleria page on polycarbonate production
- Alkylating+agents at the US National Library of Medicine Medical Subject Headings (MeSH)