Silicotungstic acid
Silicotungstic acid (also known as tungstosilicic acid) is the most commonly encountered heteropoly acid. It is a pale yellow solid with the chemical formula H4[W12SiO40]. It is used as a catalyst in the chemical industry.[1]
 | |
 | |
| Names | |
|---|---|
| Other names
Tungstosilicic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ECHA InfoCard | 100.206.333 |
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| H4[W12SiO40] | |
| Molar mass | 2878.2 g/mol |
| Melting point | 53 °C (127 °F; 326 K) |
| Structure | |
| zero | |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Related heteropoly acids |
Phosphotungstic acid |
Related compounds |
Tungsten trioxide Tungstic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
Silicotungstic acid is used to manufacture ethyl acetate by the alkylation of acetic acid by ethylene:
- C2H4 + CH3CO2H → CH3CO2C2H5
It has also been commercialized for the oxidation of ethylene to acetic acid:[1]
- C2H4 + O2 → CH3CO2H
This route is claimed as a "greener" than methanol carbonylation. The heteropoly acid is dispersed on silica gel at 20-30 wt% to maximize catalytic ability.
It has also recently been proposed as a mediator in production of hydrogen through electrolysis of water by a process that would reduce the danger of explosion while allowing efficient hydrogen production at low current densities, conducive to hydrogen production using renewable energy.[2]
Silicotungstic acid is also used for detecting nicotine and measuring its concentration.
Synthesis and structure
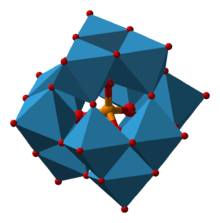
The free acid is produced by combining sodium silicate and tungsten trioxide followed treatment of the mixture with hydrochloric acid.[3] The polyoxo cluster adopts a Keggin structure, with Td point group symmetry.
References
- Misono, Makoto (2009). "Recent progress in the practical applications of heteropolyacid and perovskite catalysts: Catalytic technology for the sustainable society". Catalysis Today. 144 (3–4): 285–291. doi:10.1016/j.cattod.2008.10.054.
- Rausch, Benjamin; Symes, Mark D.; Chisholm, Greig; Cronin, Leroy (September 12, 2014). "Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting". Science. American Association for the Advancement of Science. 345 (6202): 1326–1330. Bibcode:2014Sci...345.1326R. doi:10.1126/science.1257443. PMID 25214625.
- Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY.