Daphnia
Daphnia, a genus of small planktonic crustaceans, are 0.2–5 millimetres (0.01–0.20 in) in length. Daphnia are members of the order Cladocera, and are one of the several small aquatic crustaceans commonly called water fleas because their saltatory (Wiktionary) swimming style resembles the movements of fleas. Daphnia live in various aquatic environments ranging from acidic swamps to freshwater lakes and ponds.
| Daphnia | |
|---|---|
 | |
| Daphnia sp. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Crustacea |
| Class: | Branchiopoda |
| Order: | Cladocera |
| Family: | Daphniidae |
| Genus: | Daphnia Müller, 1785 |
| Subgenera | |
| |
| Diversity | |
| > 200 spp. | |
| Synonyms [1] | |
| |
The two most readily available species of Daphnia are D. pulex (small and most common) and D. magna (large). They are often associated with a related genus in the order Cladocera: Moina, which is in the Moinidae family instead of Daphniidae and is much smaller than D. pulex (approximately half the maximum length). Daphnia eggs for sale are generally enclosed in ephippia (a thick shell, consisting of two chitinous plates, that encloses and protects the winter eggs of a cladoceran).[2]
Appearance and characteristics
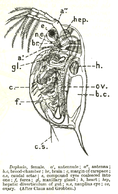
The body of Daphnia is usually 1–5 millimetres (0.04–0.20 in) long,[3] and is divided into segments, although this division is not visible.[4] The head is fused, and is generally bent down towards the body with a visible notch separating the two. In most species, the rest of the body is covered by a carapace, with a ventral gap in which the five or six pairs of legs lie.[4] The most prominent features are the compound eyes, the second antennae, and a pair of abdominal setae.[4] In many species, the carapace is translucent or nearly so and as a result they make excellent subjects for the microscope as one can observe the beating heart.[4]
Even under relatively low-power microscopy, the feeding mechanism can be observed, with immature young moving in the brood pouch; moreover, the eye being moved by the ciliary muscles can be seen, as well as blood cells being pumped around the circulatory system by the simple heart.[4] The heart is at the top of the back, just behind the head, and the average heart rate is approximately 180 bpm under normal conditions. Daphnia, like many animals, are prone to alcohol intoxication, and make excellent subjects for studying the effects of the depressant on the nervous system due to the translucent exoskeleton and the visibly altered heart rate. They are tolerant of being observed live under a cover slip and appear to suffer no harm when returned to open water.[4] This experiment can also be performed using caffeine, nicotine or adrenaline, each producing an increase in the heart rate.
Due to its intermediate size, Daphnia utilizes both diffusion and circulatory methods, producing hemoglobin in low-oxygen environments.[5]
Systematics and evolution
Daphnia is a large genus – comprising over 200 species – belonging to the cladoceran family Daphniidae.[1] It is subdivided into several subgenera (Daphnia, Australodaphnia, Ctenodaphnia), but the division has been controversial and is still in development. Each subgenus has been further divided into a number of species complexes. The understanding of species boundaries has been hindered by phenotypic plasticity, hybridization, intercontinental introductions and poor taxonomic descriptions.[6][7][8]
Ecology and behaviour

Daphnia species are normally r-selected, meaning that they invest in early reproduction and so have short lifespans. An individual Daphnia life-span depends on factors such as temperature and the abundance of predators, but can be 13–14 months in some cold, oligotrophic fish-free lakes.[9] In typical conditions, however, the life cycle is much shorter, not usually exceeding 5–6 months.[9]
Daphnia are typically filter feeders, ingesting mainly unicellular algae and various sorts of organic detritus including protists and bacteria[3][10] Beating of the legs produces a constant current through the carapace which brings such material into the digestive tract. The trapped food particles are formed into a food bolus which then moves down the digestive tract until voided through the anus located on the ventral surface of the terminal appendage.[10] The second and third pair of legs are used in the organisms' filter feeding, ensuring large unabsorbable particles are kept out, while the other sets of legs create the stream of water rushing into the organism.[10]
Swimming is powered mainly by the second set of antennae, which are larger in size than the first set.[11] The action of this second set of antennae is responsible for the jumping motion.[11]
Daphnia are known to show behavioral changes or modifications to their morphology in the presence of predator kairomones (chemical signals). Some examples of morphological modifications include larger size at hatching, increased bulkiness, and the development of “neck-teeth". For example, juveniles of D. pulex will have a larger size after hatching, along with developing neck-teeth at the back of the head, when in the presence of Chaoborus kairomones. These morphological defenses have shown to reduce mortality due to Chaoborus predation, which is a gape-limited predator. Chitin-related genes (deacetylases) are thought to play an important part in the expression/development of these morphological defenses in Daphnia. Chitin-modifying enzymes (chitin deacetylases) have been shown to catalyse the N-deacetylation of chitin to influence the protein-binding affinity of these chitin filaments. [12]
Life cycle


Most Daphnia species have a life cycle based on "cyclical parthenogenesis", alternating between parthenogenetic (asexual) reproduction and sexual reproduction.[3] For most of the growth season, females reproduce asexually. They produce a brood of diploid eggs every time they moult; these broods can contain as few as 1–2 eggs in smaller species, such as D. cucullata, but can be over 100 in larger species, such as D. magna. Under typical conditions, these eggs hatch after a day, and remain in the female's brood pouch for around three days (at 20 °C). They are then released into the water, and pass through a further 4–6 instars over 5–10 days (longer in poor conditions) before reaching an age where they are able to reproduce.[3] The asexually produced offspring are typically female.
Towards the end of the growing season, however, the mode of reproduction changes, and the females produce tough "resting eggs" or "winter eggs".[3] When environmental condition deteriorate (e.g. crowding), some of the asexually produced offspring develop into males.[3] The females start producing haploid sexual eggs, which the males fertilise. In species without males, resting eggs are also produced asexually and are diploid. In either case, the resting eggs are protected by a hardened coat called the ephippium, and are cast off at the female's next moult. The ephippia can withstand periods of extreme cold, drought or lack of food availability, and hatch – when conditions improve – into females (They are close to being classed as extremophiles) .[3]
Conservation
Several Daphnia species are considered threatened. The following are listed as vulnerable by IUCN: Daphnia nivalis, Daphnia coronata, Daphnia occidentalis, and Daphnia jollyi. Some species are halophiles, and can be found in hypersaline lake environments, an example of which is the Makgadikgadi Pan.[13]
Uses
Daphnia spp. are a popular live food in tropical and marine fish keeping. They are often fed to tadpoles or small species of amphibians such as the African dwarf frog (Hymenochirus boettgeri).
Daphnia may be used in certain environments to test the effects of toxins on an ecosystem, which makes them an indicator genus, particularly useful because of its short lifespan and reproductive capabilities. Because they are nearly transparent, their internal organs are easy to study in live specimens (e.g. to study the effect of temperature on the heart rate of these ectothermic organisms). Daphnia is also commonly used for experiments to test climate change aspects, as ultraviolet radiation (UVR) that seriously damage zooplankton species (e.g. decrease feeding activity[14]).
Because of their thin membrane, which allows drugs to be absorbed, they are used to monitor the effects of certain drugs, such as adrenaline or capsaicin, on the heart.
Invasive species
Some species of daphnia have developed permanent, non-temporary defenses against fish eating them such as spines and long hooks on the body which also cause them to become entangled on fishing lines and cloud water with their high numbers. Species such as Daphnia lumholtzi[15][16][17][18] (native to east Africa, the Asian subcontinent of India, and east Australia) have these characteristics and great care should be taken to prevent them from spreading further in North American waters.
Some species of daphnia native to North America can develop sharp spines at the end of the body and helmet-like structures on the head when they detect predators,[19][20] but this is overall temporary for such daphnia species and they do not completely overwhelm or discourage native predators from eating them. While daphnia are an important base of the food chain in freshwater lakes (and vernal pools), they become a nuisance when they are unable to be eaten by native macroscopic predators and there is some concern that the original spineless and hookless water fleas and daphnia end up out-competed by the invasive ones. (This may not be the case, however, and the new invaders may mostly be a tangling and clogging nuisance.)
See also
- List of Daphnia species
- Moina, which are sometimes referred to as Daphnia
- Rotifer
- Zooplankton
- Crustacean
References
- A. Kotov; L. Forró; N. M. Korovchinsky; A. Petrusek (March 2, 2012). "Crustacea-Cladocera checkList" (PDF). World checklist of freshwater Cladocera species. Belgian Biodiversity Platform. Retrieved October 29, 2012.
- N.N.Smirnov (2014). The physiology of the Cladocera. Amsterdam: Academic Press.
- Dieter Ebert (2005). "Introduction to Daphnia biology". Ecology, Epidemiology, and Evolution of Parasitism in Daphnia. Bethesda, MD: National Center for Biotechnology Information. ISBN 978-1-932811-06-3.
- "Daphnia". Oneida Lake Education Initiative. Stony Brook University. Retrieved October 9, 2013.
- van Bergen, Yfke (2004). "DAPHNIA BREATHES EASY". biologists.org. Retrieved 2018-04-11.
- Niles Lehman; Michael E. Pfrender; Phillip A. Morin; Teresa J. Crease; Michael Lynch (1995). "A hierarchical molecular phylogeny within the genus Daphnia". Molecular Phylogenetics and Evolution. 4 (4): 395–407. doi:10.1006/mpev.1995.1037. PMID 8747296.
- Derek J. Taylor; Paul D. N. Hebert; John K. Colbourne (1996). "Phylogenetics and evolution of the Daphnia longispina group (Crustacea) based on 12S rDNA sequence and allozyme variation" (PDF). Molecular Phylogenetics and Evolution. 5 (3): 495–510. doi:10.1006/mpev.1996.0045. PMID 8744763. Archived from the original (PDF) on 2007-04-18.
- Sarah J. Adamowicz; Paul D. N. Hebert; María Christina Marinone (2004). "Species diversity and endemism in the Daphnia of Argentina: a genetic investigation". Zoological Journal of the Linnean Society. 140 (2): 171–205. doi:10.1111/j.1096-3642.2003.00089.x.
- Barbara Pietrzak; Anna Bednarska; Magdalena Markowska; Maciej Rojek; Ewa Szymanska; Miroslaw Slusarczyk (2013). "Behavioural and physiological mechanisms behind extreme longevity in Daphnia". Hydrobiologia. 715 (1): 125–134. doi:10.1007/s10750-012-1420-6.
- Z. Maciej Gliwicz (2008). "Zooplankton". In Patrick O'Sullivan; C. S. Reynolds (eds.). The Lakes Handbook: Limnology and Limnetic Ecology. John Wiley & Sons. pp. 461–516. ISBN 978-0-470-99926-4.
- Stanley L. Dodson; Carla E. Cáceres; D. Christopher Rogers (2009). "Cladocera and other Branchiopoda". In James H. Thorp; Alan P. Covich (eds.). Ecology and Classification of North American Freshwater Invertebrates (3rd ed.). Academic Press. pp. 773–828. ISBN 978-0-08-088981-8.
- Christjani, Mark (2016). "Phenotypic plasticity in three Daphnia genotypes in response to predator kairomone: evidence for an involvement of chitin deacetylases". The Journal of Experimental Biology.
- C. Michael Hogan (2008). "Makgadikgadi". The Megalithic Portal.
- Fernández, Carla Eloisa; Rejas, Danny (2017-04-05). "Effects of UVB radiation on grazing of two cladocerans from high-altitude Andean lakes". PLOS One. 12 (4): e0174334. doi:10.1371/journal.pone.0174334. ISSN 1932-6203. PMC 5381789. PMID 28379975.
- USGS: Nonindigenous Aquatic Species: Daphnia lumholtzi
- Center for Freshwater Biology - University of New Hampshire: Daphnia lumholtzi
- ISSG: Global Invasive Species Database: Daphnia lumholtzi (crustacean)
- James A. Stoeckel, Illinois Natural History Survey: Daphnia lumholtzi: The Next Great Lakes Exotic? Archived 2016-12-20 at the Wayback Machine
- Elizabeth A. Colburn (2004), Vernal Pools: Natural History and Conservation, page 118 of paperback second edition from 2008
- Patrick Lavens and Patrick Sorgeloos, Manual on the Production and Use of Live Food for Aquaculture: Daphnia and Moina
External links
| Wikimedia Commons has media related to Daphnia. |
