D-Amino acid
D-Amino acids are amino acids where the stereogenic carbon alpha to the amino group has the D-configuration. For most naturally-occurring amino acids, this carbon has the L-configuration. D-Amino acids are most occasionally found in nature as residues in proteins. They are formed from ribosomally-derived D-amino acid residues.
Structure and general properties
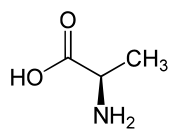 D-alanine.
D-alanine.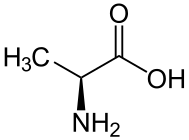 L-alanine.
L-alanine.
L- and D-amino acids are usually enantiomers. The exceptions are two amino acids with two stereogenic centers, threonine and isoleucine. Aside from those two special cases, L- and D-amino acids have identical properties (color, solubility, melting point) under many conditions. In the biological context however, which is chiral, these enatiomers can behave very differently. Thus, D-amino acids have low nutritional value, in part because they are not digested well.[1]
Biosynthesis
Two enzymes convert L-amino acids to D-amino acids. D-Amino-acid racemase, a PLP-dependent enzyme, racemizes amino acids via the formation of the alpha-iminoacids, where the stereogenic center is lost. L-amino-acid oxidases convert L-amino acids to the alpha-ketoacids, which are susceptible to reductive amination. Some amino acids are prone to racemization, one example being lysine, which racemizes via formation of pipecolic acid.
In peptides, L-amino acid residues slowly racemize, resulting in the formation of some D-amino acid residues. Racemization occurs via deprotonation of the methyne that is alpha to the amido group. Rates increase with pH.
Many D-amino acids found in higher organisms are derived from microbial sources. The D-alanine in peptidoglycans that comprise bacterial cell walls helps its host resist attack by proteolytic enzymes. Several antibiotics, e.g. bacitracin, contain D-amino acid residues.[1]
References
- Mendel Friedman (1999). "Chemistry, Nutrition, and Microbiology of D-Amino Acids". J. Agric. Food Chem. 47 (9): 3457–79. doi:10.1021/jf990080u. PMID 10552672.
