Cyathus stercoreus
Cyathus stercoreus, commonly known as the dung-loving bird's nest,[2] is a species of fungus in the genus Cyathus, family Nidulariaceae. Like other species in the Nidulariaceae, the fruiting bodies of C. stercoreus resemble tiny bird's nests filled with eggs. The fruiting bodies are referred to as splash cups, because they are developed to use the force of falling drops of water to dislodge and disperse their spores. The species has a worldwide distribution, and prefers growing on dung, or soil containing dung; the specific epithet is derived from the Latin word stercorarius, meaning "of dung".[3]
| Cyathus stercoreus | |
|---|---|
| Scientific classification | |
| Kingdom: | |
| Division: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | C. stercoreus |
| Binomial name | |
| Cyathus stercoreus (Schwein.) De Toni (1888) | |
| Synonyms | |
|
Several, including:
| |
Description
The fruiting bodies, or perida, are funnel- or barrel-shaped, 6–15 mm tall, 4–8 mm wide at the mouth, sometimes short-stalked, golden brown to blackish brown in age.[4] The outside wall of the peridium, the ectoperidium, is covered with tufts of fungal hyphae that resembles shaggy, untidy hair. However, in older specimens this outer layer of hair (technically a tomentum) may be completely worn off. The internal wall of the cup, the endoperidium, is smooth and grey to bluish-black. The 'eggs' of the bird's nest – the peridioles – are blackish, 1–2 mm in diameter,[4] and there are typically about 20 in the cup.[5] Peridioles are often attached to the fruiting body by a funiculus, a structure of hyphae that is differentiated into three regions: the basal piece, which attaches it to the inner wall of the peridium, the middle piece, and an upper sheath, called the purse, connected to the lower surface of the peridiole. In the purse and middle piece is a coiled thread of interwoven hyphae called the funicular cord, attached at one end to the peridiole and at the other end to an entangled mass of hyphae called the hapteron. However, Brodie reports that sometimes C. stercoreus is found without a funiculus, which has led some authors to misidentify this species with the genus Nidula.[6]
The spores of C. stercoreus are roughly spherical and relatively large, with typical dimensions of 20–35 x 20–25 µm,[4] although great variability in spore size has been noted.[6] The spores are sessile (growing directly from the surface of the basidium, without attachment via a sterigmata), and are separated from the basidia after it collapses and gelatinizes. This is accompanied by the gelatinization of the inner walls of the peridiole.[7]
Ultrastructure
Examination of fruiting bodies using scanning electron microscopy and transmission electron microscopy has revealed details about their ultrastructure—their microscopic architecture and arrangement. For example, the hyphae of the hapteron form a dense tangled network, while the hyphae of the funicular cord are arranged in a twisted form like a rope.[5] Further, the funicular cord, known to be highly elastic and with a high tensile strength, is made of thicker hyphae than the rest of the funiculus.[5] Also, the ecto- and endoperidium are made of thick-walled, unbranched hyphae, known as skeletal hyphae. It has been proposed that these skeletal hyphae form a structural network that helps the fruiting body maintain the elasticity vital for proper functioning of the spore dispersal mechanism.[5]
Life cycle
The life cycle of Cyathus stercoreus, which contains both haploid and diploid stages, is typical of taxa in the basidiomycetes that can reproduce both asexually (via vegetative spores), or sexually (with meiosis). Basidiospores produced in the peridioles each contain a single haploid nucleus. After dispersal, the spores germinate and grow into homokaryotic hyphae, with a single nucleus in each compartment. When two homokaryotic hyphae of different mating compatibility groups fuse with one another, they form a dikaryotic (containing two nuclei) mycelia in a process called plasmogamy. After a period of time (approximately 40 days when grown from pure culture in the laboratory)[8] and under the appropriate environmental conditions, fruiting bodies may be formed from the dikaryotic mycelia. These fruiting bodies produce peridioles containing the basidia upon which new basidiospores are made. Young basidia contain a pair of haploid sexually compatible nuclei which fuse, and the resulting diploid fusion nucleus undergoes meiosis to produce haploid basidiospores.[9]
Development
Extreme variability in fruiting body form and color has been noted for C. stercoreus.[10] Brodie reported discovering a slender-stemmed "twinned" form, with two fruiting bodies originating from the same stalk.[11] As has been shown in laboratory-grown specimens, the development and form of the fruiting bodies is at least partially dependent on the intensity of light it receives during development. For example, exposure of the heterokaryotic mycelium to light is required for fruiting to occur, and furthermore, this light needs to be at a wavelength of less than 530 nm.[12] Lu suggests that certain growing conditions – such as a shortage in available nutrients – shifts the fungus' metabolism to produce a hypothetical "photoreceptive precursor" that enables the growth of the fruiting bodies to be stimulated and affected by light.[13] The fungi is also positively phototrophic, that is, it will orient its fruiting bodies in the direction of the light source.[14]
Habitat and distribution
Being coprophilous, C. stercoreus grows on dung, in soil with dung, and bonfire sites; it has also been recorded growing on sand dunes.[4] The fungus is known to have a worldwide distribution, and Curtis Gates Lloyd, in his monograph on the Nidulariaceae, wrote that it "probably occurs in every country where manure occurs".[15]
Spore dispersal
When a drop of water hits the interior of the cup at the appropriate angle and velocity, the peridioles are ejected into the air by the force of the drop. The force of ejection tears open the purse, and results in the expansion of the funicular cord, formerly coiled under pressure in the lower part of the purse. The peridioles, followed by the highly adhesive funicular cord and basal hapteron, may hit a nearby plant stem or stick. The hapteron sticks to it, and the funicular cord wraps around the stem or stick powered by the force of the still-moving peridiole. After drying out, the peridiole remains attached to the vegetation, where it may be eaten by a grazing herbivorous animal, and later deposited in that animal's dung to continue the life cycle.[16]
Bioactive compounds
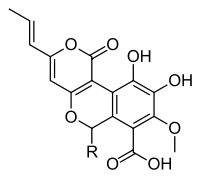
A number of polyketide-type antioxidative compounds, cyathusals A, B, and C, and pulvinatal have been isolated and identified from the liquid culture of Cyathus stercoreus.[17] Furthermore, the polyketides known as cyathuscavin A, B, and C (isolated from liquid culture) also have antioxidant activity, and have DNA protection activity.[18]
Uses
While inedible,[19] the species has other uses.
Traditional medicine
In Traditional Chinese medicine, a decoction of this fungus is used to help relieve the symptoms of gastralgia, or stomach ache.[20]
Agricultural and industrial
Cyathus stercoreus has been investigated for its ability to break down lignin and cellulose in agricultural byproducts, like wheat straw or grasses.[21][22][23] It selectively breaks down lignin, leaving much of the cellulose intact, which increases the amount of digestible carbohydrate for ruminant mammals, and enhances both its value as a food source and its biodegradability.[24] The enzymes responsible, laccase and manganese peroxidase, also have industrial applications for lignin degradation and removal in the pulp and paper industry.[25] Liquid cultures of C. stercoreus have also been shown to biodegrade the explosive compound 2,4,6-trinitrotoluene (TNT).[26]
See also
References
- Speg., Anales del Museo Nacional de Historia Natural Buenos Aires 6: 185 (1898)
- Emberger G. "Cyathus stercoreus". Retrieved 2009-03-03.
- Stearn WT (2004). Botanical Latin. Timber Press (OR). p. 403. ISBN 0-88192-627-2. Google Books
- Ellis JB, Ellis MB (1990). Fungi without gills (Hymenomycetes and Gasteromycetes): An identification handbook. London: Chapman and Hall. p. 225. ISBN 0-412-36970-2.
- Flegler SL, Hooper GR. (1978). "Ultrastructure of Cyathus stercoreus".Mycologia 70(6): 1181–1190.
- Brodie, The Bird's Nest Fungi, p. 168.
- Martin GW. (1927). "Basidia and spores of the Nidulariaceae". Mycologia 19(5): 239–47.
- Brodie, The Bird's Nest Fungi, p. 10.
- Deacon J. (2005). Fungal Biology. Cambridge, MA: Blackwell Publishers. pp. 31–32. ISBN 1-4051-3066-0.
- Brodie HJ. (1948). "Variation in the fruit bodies of Cyathus stercoreus produced in culture". Mycologia 40: 614–26.
- Brodie HJ (1977). "Twin fruit bodies in a slender-stemmed form of Cyathus stercoreus". Mycologia. Mycological Society of America. 69 (1): 199–203. doi:10.2307/3758634. JSTOR 3758634.
- Garnett E. (1958). "Studies of factors affecting fruiting body formation in Cyathus stercoreus (Schw.) de Toni". PhD Dissertation, Indiana University.
- Lu B. (1965). "The role of light in fructification of the basidiomycete Cyathus stercoreus". American Journal of Botany 52: 432–437.
- Brodie, The Birds Nest Fungi, p. 57–58.
- Gates CL. (1906). "The Nidulariaceae". Mycological Writings 2: 1–30.
- Brodie, The Bird's Nest Fungi, pp. 7–9.
- Kang HS, Jun EM, Park SH, Heo SJ, Lee TS, Yoo ID, Kim JP (2007). "Cyathusals A, B, and C, antioxidants from the fermented mushroom Cyathus stercoreus". Journal of Natural Products. 70 (6): 1043–1045. doi:10.1021/np060637h. PMID 17511503.
- Kang HS, Kim KR, Jun EM, Park SH, Lee TS, Suh JW, Kim JP (2008). "Cyathuscavins A, B, and C, new free radical scavengers with DNA protection activity from the Basidiomycete Cyathus stercoreus". Bioorganic & Medicinal Chemistry Letters. 18 (14): 4047–4050. doi:10.1016/j.bmcl.2008.05.110. PMID 18565749.
- Phillips, Roger (2010). Mushrooms and Other Fungi of North America. Buffalo, NY: Firefly Books. p. 341. ISBN 978-1-55407-651-2.
- Bo L, Bu Y-S. Fungi Pharmacopoeia (Sinica). The Kinoko Company: Oakland, California. p. 246.
- Wikclow DT, Detroy RW, Jessee BA (1980). "Decomposition of lignocellulose by Cyathus stercoreus (Schw.) de Toni NRRL 6473, a "white rot" fungus" from cattle dung". Applied and Environmental Microbiology. 40 (1): 169–170. PMC 291542. PMID 16345591.
- Halsall DM (1993). "Inoculation of wheat straw to enhance lignocellulose breakdown and associated nitrogenase activity". Soil Biology and Biochemistry. 25 (4): 419. doi:10.1016/0038-0717(93)90067-L.
- Akin DE, Rigsby LL, Sethuraman A, Morrison WH, Gamble GR, Eriksson KEL (1995). "Alterations in structure, chemistry, and biodegradability of grass lignocellulose treated with the white-rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus". Applied and Environmental Chemistry. 61: 1591–98. PMC 167414. PMID 7747973.
- Abbott TP, Wicklow DT (1984). "Degradation of lignin by Cyathus species" (PDF). Applied and Environmental Microbiology. 47 (3): 585–587. PMC 239724. PMID 16346497. Archived from the original (PDF) on 2011-07-21.
- Sethuramin A, Akin DE, Eriksson KEL (1999). "Production of ligninolytic enzymes and synthetic lignin mineralization by the bird's nest fungus Cyathus stercoreus". Applied Microbiology and Biotechnology. 52 (5): 689–697. doi:10.1007/s002530051580. PMID 10570816.
- Chen J. (1995). "Development of fungal degrading system to detoxify 2,4,6-trinitrotoluene (TNT) in liquid phase bioreactors". PhD dissertation, Texas A&M University. 127 pp.
Cited text
Brodie HJ (1975). The Bird's Nest Fungi. Toronto: University of Toronto Press. ISBN 0-8020-5307-6.
External links
