Cuprate
Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ([CuCl4]2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate [Cu(CH3)2]−).[1] The term cuprates derives from the Latin word for copper, cuprum. The term is mainly used in three contexts - oxide materials, anionic coordination complexes, and anionic organocopper compounds.
Oxides
One of the simplest oxide-based cuprates is the copper(III) oxide KCuO2. This species can be viewed as the K+ salt of the polyanion [CuO−
2]n. As such the material is classified as a cuprate. This dark blue diamagnetic solid is produced by heating potassium peroxide and copper(II) oxide in an atmosphere of oxygen:[2]
- K2O2 + 2 CuO → 2 KCuO2
Coordination complexes
Copper forms many anionic "cuprate" coordination complexes with negatively charged ligands such as cyanide, hydroxide, and halides. Copper(I) derivatives tend to be colorless, copper(II) complexes are often turquoise-blue, and copper(III) and copper(IV) complexes are often orange-red.[3]
.png)
One example of a copper(I)-based cuprate is the tetrahedral complex tetracyanocuprate(I), [Cu(CN)4]3−.[4]
Copper(II) anions are most common, especially the chlorocuprates, such as trichlorocuprate(II) [CuCl3]−, tetrachlorocuprate(II) [CuCl4]2− and pentachlorocuprate(II) [CuCl5]3−.[1] The light blue solid sodium tetrahydroxycuprate is well known; it is prepared by heating cupric hydroxide with concentrated sodium hydroxide.[5]
- Cu(OH)2 + 2 NaOH → Na2Cu(OH)4
Dilithium tetrachlorocuprate (Li2CuCl4) is an effective catalyst for the couplings of Grignard reagents. It is prepared by mixing lithium chloride (LiCl) and copper(II) chloride (CuCl2) in tetrahydrofuran.[6]
There are also rare copper(III) and copper(IV) complexes such as the hexafluorocuprate(III) [CuF6]3− and hexafluorocuprate(IV) [CuF6]2−, which are strong oxidizing agents.
Organic cuprates
Cuprates have a role in organic synthesis. Organic cuprates often have the formula [CuR2]− or [CuR3]2−, where R is an alkyl or aryl. These reagents find use as nucleophilic alkylating reagents. In stark contrast to the oxidic cuprates, the handling of these organocopper requires air-free techniques.[7]
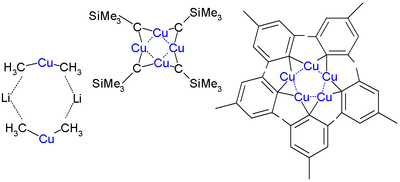 Organocopper aggregates containing more than one copper atom |
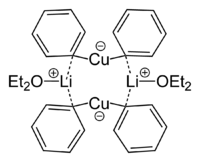 Skeletal formula of a dimer from the crystal structure of lithium diphenylcuprate etherate, 2Ph2CuLi·2OEt2[8] |
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- G. Brauer, ed. (1963). "Potassium Cuprate (III)". Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). NY: Academic Press. p. 1015.
- Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. pp. 1252–1264. ISBN 0-12-352651-5.
- "Sodium Tetrahydroxocuprate (II) in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1015.
- Atta-ur-Rahman (2002). Bioactive natural products. Elsevier. pp. 73, 81, 83. ISBN 0-444-51004-4.
- Louis S. Hegedus (1999). Transition metals in the synthesis of complex organic molecules. University Science Books. pp. 61–65. ISBN 1-891389-04-1.
- Lorenzen, Nis Peter; Weiss, Erwin (1990). "Synthesis and Structure of a Dimeric Lithium Diphenylcuprate:[{Li(OEt2)}(CuPh2)]2". Angewandte Chemie International Edition in English. 29 (3): 300. doi:10.1002/anie.199003001.