Coronin
Coronin is an actin binding protein which also interacts with microtubules and in some cell types is associated with phagocytosis.[1][2][3] Coronin proteins are expressed in a large number of eukaryotic organisms from yeast to humans.
Discovery
Initially, a 55 kDa protein was isolated from the actomyosin complex of Dictyostelium discoideum, which was later shown to bind actin in vitro.[4] This actin binding protein was named coronin after its strong immunolocalisation in the actin rich crown like extension of the cell cortex in D. discoideum. Initially this protein was admitted into club of actin binding proteins with least enthusiasm, as the primary structure did not match any other ABPs. But null mutation of coronin in D. discoideum resulted in impaired cytokinesis, and many actin mediated processes like endocytosis, cell motility etc. Later on, the protein was identified in many eukaryotic cells.[5]
Structure
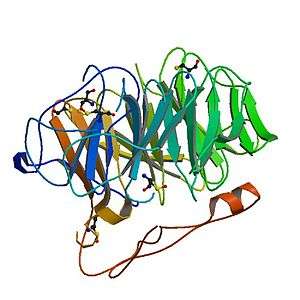
Coronin belongs to the WD-repeat containing proteins, which form a beta propeller tertiary structure. The crystal structure of coronin 1A (see figure to the right) containing major part of the protein was solved in 2006.[6]
The WD-repeat is a structural motif comprising approximately 40 amino acids usually ending with the amino acid sequence tryptophan (W) – aspartic acid (D) and hence the name WD.[7]
WD-40-domain –repeat proteins are defined by the presence of at least four WD repeats located centrally in the protein. These domains were discovered in 1986 and are characterized by a partial conserved domain of 40-60 amino acids, starting with GH dipeptide 11-24 residue away from the N-terminus and ending with a tryptophane-aspartic acid (WD) dipeptide at the C-terminus. The WD domain has no intrinsic catalytic activity and is thought to serve as a stable platform for simultaneous interaction. WD repeat proteins have diverse cellular functions. They play a central role in physiological processes like signal transduction, transcriptional regulation, cytoskeleton remodeling, and regulation of vesicle trafficking.
Coronin homologues both in vertebrates and invertebrates forms a subfamily among WD repeat proteins. Coronin contains 3-5 WD clustered repeats forming the central core domain. Apart from the core domain, almost all coronins have a short conserved N-terminal motif and coiled coil motif of 50 amino acids at the C-terminus. The N-terminal region contains 12 basic amino acids which can be taken as signature as it is present in only coronin proteins. A recent study shows that these basic residues are involved in actin binding. Furthermore, each coronin contains a unique divergent region between the WD domain and C-terminal coiled coil region. The number of amino acids in this region varies greatly. The unique region of Dictyostelium has 22 amino acids whereas mammalian coronins contains about 50 amino acids. The coronin-like proteins from budding yeast Crn1 and one of the coronins in Caenorhabditis elegans has a much longer unique region i.e. 194 vs 144aa. The unique region of yeast coronin shows homologies with microtubule binding domains of the MAPs and yeast coronin binds both actin and microtubule and serve as bridge between them.
A second region of variability exists in the fourth β-strand of the third WD repeats.
Function
Yeast coronin Crn1[8] and Drosophila Dpod1 were found to crosslink the actin and microtubule cytoskeleton. Caenorhabditis elegans POD-1 and Drosophila coronin homologue regulate the actin cytoskeleton and are involved in vesicular trafficking.
Seven different isoforms of coronin have been reported in mammals. The most well-studied isoforms are coronin 1 (Coronin 1A) and coronin 1B. Coronin 1 exerted an inhibitory effect on cellular steady-state F-actin formation via an Arp2/3-dependent mechanism. Whereas coronin 1 was required for chemokine-mediated migration, it was dispensable for T cell antigen receptor functions in T cells.[9] Coronin 1B is required for efficient cell protrusion and migration.[10][11] Recent study demonstrates that coronin 1B inhibits the Arp2/3 complex activity by replacing it at the branched actin structure.[12] Mammalian Coronin-7 does not interact with actin nor does it execute any actin mediated processes, but rather participates in Golgi trafficking.
Although coronin is present in almost all eukaryotic organisms and has different functions, these proteins have all been shown to bind F-actin and localize in the dynamic F-actin rich area of cells. Recent study shows that coronin prefers ATP/ADP-Pi containing F-actin over ADP containing F-actin, which might explain their unique cellular localization.[13]
Classification
- Short conventional coronins. Contain 450-650 amino acids with C-terminus coiled coil region of 30-40 amino acids that mediates homophilic dimerization and/or olimerization of coronins (e.g., CRN1, CRN2).
- Long coronins. Two core domains with no C-terminal coiled coil region.
N-terminus signature region is reduced to 5aa and appears in front of each WD-repeat core domain (e.g., CRN7, POD-1)
Family members
Human proteins which are members of the coronin family include:
References
- Uetrecht AC, Bear JE (June 2006). "Coronins: the return of the crown". Trends Cell Biol. 16 (8): 421–6. doi:10.1016/j.tcb.2006.06.002. PMID 16806932.
- de Hostos EL (September 1999). "The coronin family of actin-associated proteins". Trends Cell Biol. 9 (9): 345–50. doi:10.1016/S0962-8924(99)01620-7. PMID 10461187.
- Rybakin V, Clemen CS (June 2005). "Coronin proteins as multifunctional regulators of the cytoskeleton and membrane trafficking". BioEssays. 27 (6): 625–32. doi:10.1002/bies.20235. PMID 15892111.
- de Hostos EL, Bradtke B, Lottspeich F, Guggenheim R, Gerisch G (December 1991). "Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits". EMBO J. 10 (13): 4097–104. PMC 453159. PMID 1661669.
- de Hostos EL (2008). "A brief history of the coronin family". Subcell. Biochem. 48: 31–40. doi:10.1007/978-0-387-09595-0_4. PMID 18925369.
- PDB: 2AQ5; Appleton BA, Wu P, Wiesmann C (January 2006). "The crystal structure of murine coronin-1: a regulator of actin cytoskeletal dynamics in lymphocytes". Structure. 14 (1): 87–96. doi:10.1016/j.str.2005.09.013. PMID 16407068.
- Li D, Roberts R (December 2001). "WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases". Cell. Mol. Life Sci. 58 (14): 2085–97. doi:10.1007/PL00000838. PMID 11814058.
- Humphries CL, Balcer HI, D'Agostino JL, Winsor B, Drubin DG, Barnes G, Andrews BJ, Goode BL (December 2002). "Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin". J. Cell Biol. 159 (6): 993–1004. doi:10.1083/jcb.200206113. PMC 2173993. PMID 12499356.
- Föger N, Rangell L, Danilenko DM, Chan AC (August 2006). "Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis". Science. 313 (5788): 839–42. doi:10.1126/science.1130563. PMID 16902139.
- Cai L, Marshall TW, Uetrecht AC, Schafer DA, Bear JE (May 2007). "Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge". Cell. 128 (5): 915–29. doi:10.1016/j.cell.2007.01.031. PMC 2630706. PMID 17350576.
- Cai L, Holoweckyj N, Schaller MD, Bear JE (September 2005). "Phosphorylation of coronin 1B by protein kinase C regulates interaction with Arp2/3 and cell motility". J Biol Chem. 280 (36): 31913–23. doi:10.1074/jbc.M504146200. PMID 16027158.
- Cai L, Makhov AM, Schafer DA, Bear JE (September 2008). "Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia". Cell. 134 (5): 828–42. doi:10.1016/j.cell.2008.06.054. PMC 2570342. PMID 18775315.
- Cai L, Makhov AM, Bear JE (May 2007). "F-actin binding is essential for coronin 1B function in vivo". J Cell Sci. 120 (10): 1779–90. doi:10.1242/jcs.007641. PMID 17456547.
Further reading
- Rosentreter A, Hofmann A, Xavier CP, et al. (2007). "Coronin 3 involvement in F-actin-dependent processes at the cell cortex". Exp. Cell Res. 313 (5): 878–95. doi:10.1016/j.yexcr.2006.12.015. hdl:10072/14982. PMID 17274980.
- Rush J, Moritz A, Lee KA, et al. (2005). "Immunoaffinity profiling of tyrosine phosphorylation in cancer cells". Nat. Biotechnol. 23 (1): 94–101. doi:10.1038/nbt1046. PMID 15592455.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Spoerl Z, Stumpf M, Noegel AA, Hasse A (2003). "Oligomerization, F-actin interaction, and membrane association of the ubiquitous mammalian coronin 3 are mediated by its carboxyl terminus". J. Biol. Chem. 277 (50): 48858–67. doi:10.1074/jbc.M205136200. PMID 12377779.
- Iizaka M, Han HJ, Akashi H, et al. (2000). "Isolation and chromosomal assignment of a novel human gene, CORO1C, homologous to coronin-like actin-binding proteins". Cytogenet. Cell Genet. 88 (3–4): 221–4. doi:10.1159/000015555. PMID 10828594.
- Okumura M, Kung C, Wong S, et al. (1998). "Definition of family of coronin-related proteins conserved between humans and mice: close genetic linkage between coronin-2 and CD45-associated protein". DNA Cell Biol. 17 (9): 779–87. doi:10.1089/dna.1998.17.779. PMID 9778037.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Wick M, Bürger C, Brüsselbach S, et al. (1994). "Identification of serum-inducible genes: different patterns of gene regulation during G0-->S and G1-->S progression". J. Cell Sci. 107 (1): 227–39. PMID 8175911.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.