CORO1A
Coronin-1A is a protein that in humans is encoded by the CORO1A gene.[5][6] It has been implicated in both T-cell mediated immunity and mitochondrial apoptosis. In a recent genome-wide longevity study, its expression levels were found to be negatively associated both with age at the time of blood sample and the survival time after blood draw.[7]
Discovery
The coronin protein family was discovered in 1991 by Eugenio L. Hostos. Hostos used a cytoskeletal preparation called the “contracted propeller” that efficiently helped with the purification of cytoskeletal proteins. This technique allowed him to precipitate actomyosin components together with the desired proteins.[8]
These protein were named Corona, which is the Latin word for crown, because of the crown-like shape that it forms when making contact with the surface of the cell. Coronin-1a has been the most researched one due to its complexity and intriguing structural components. After research, it was determined that coronin-1a serves as actin binding facilitator when reacted with K-glutamate. The anion K + and glutamate were used because of it similarity to the environment inside the cell, allowing coronin-1a to bind to F-actin.
Later on, the complementary DNA (cDNA) of coronin-1a was cloned in an expression library, this led to the conclusion that coronin-1a has very similar structure to the beta (β) subunits of the G proteins (Gβ). Therefore, it was established that coronin-1a has five WD motif repeats, and this repeats seven times forming a propeller like structure.[8]
In the cell, coronin-1a serves as an auxiliary to many cytoskeletal process that involve actin. It was concluded that coronin-1a is known to affect the “cytoskeletal reorganization” as well “actin dynamics” together with other protein. [8]
Phylogeny
The coronin family is composed of twelve subfamilies which include: seven subfamilies that fall under vertebrates and five subfamilies that are composed of metazoans, fungi and amoeba.
The evolutionary coronin subfamilies have been grouped by its similarities and relationships between the different proteins. Coronin-1a (also referenced as CORO1A, Coronin 4 and CRN4) has been found in 19 vertebrates.[9]
Function

Coronin-1a has been found in the cell cortex of macrophages, which are white blood cells, helping with a process called phagocytosis. The model on Figure 3 shows coronin-1a’s involvement in macrophages. When the cell is at rest, Coronin-1a is spread out throughout the cytoplasm and the cell cortex. Therefore, when a pathogen enters the cell, Coronin-1a binds to phagosomal membrane making sure of the binding and activation of calcinuerin, this resulting in a stop of fusion lysosomes with phagosomes. In other words, if coronin-1a is removed and calcinuerin is inhibited then it allows the initiation of the fusion of phagosomes with lysosome and the killing of mycobacteria.[10]
The phylogenetic tree of the coronin family it is broad. The same way that coronin-1a helps with the reorganization of the cytoskeleton and dynamic activity with other proteins in vertebrates, Coronin can also be seen in non-vertebrates, for example in Toxoplasma gondii (also known as TgCor).[11]
Toxoplasma gondii coronin (TgCor) binds to F-actin and it accelerates the actin polymerization process. It also prevents incursions and exits. As well as every other coronin, TgCor is an actin binding protein, it delocalizes to the posterior side of invading parasites and blocks them from leaving.[11]
Structure
The structure of aoronin-1A is made out of five WD repeats, and this motifs repeat seven time forming a propeller like structures.
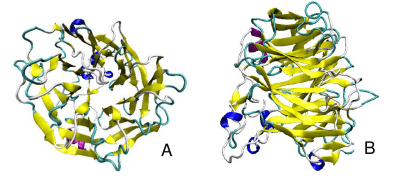
The new ribbon visualization of the secondary structure of coronin-1a. In model A, is the front view of coronin-1a, the secondary structure allows you to clearly see the parallel beta sheets moving towards the bottom of the structure. Model B, is the side view of the protein which shows the turns and the coils between the beta sheets. From this pictures we are able to see that the alpha helix and helix strands are concentrated at the bottom of the protein.[12]
.png)
Coronin-1a was input into Database of Secondary Structure Program, where the Protein Data Bank database entered and a secondary structure panel is designed where one is clearly able to see the seven repeat that makes the propeller. Also, it displays the amino acid sequence of coronin-1a. The yellow arrows mean the beta strands, the purple loops are the turns, the black lines means empty meaning that there was no secondary structure assigned, the light pink is 3/10-helix is formed, royal blue line is a bend and finally the red helix signifies the alpha helices.
References
- GRCh38: Ensembl release 89: ENSG00000102879 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000030707 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Okumura M, Kung C, Wong S, Rodgers M, Thomas ML (Sep 1998). "Definition of family of coronin-related proteins conserved between humans and mice: close genetic linkage between coronin-2 and CD45-associated protein". DNA and Cell Biology. 17 (9): 779–87. doi:10.1089/dna.1998.17.779. PMID 9778037.
- "Entrez Gene: CORO1A coronin, actin binding protein, 1A".
- Kerber RA, O'Brien E, Cawthon RM (Jun 2009). "Gene expression profiles associated with aging and mortality in humans". Aging Cell. 8 (3): 239–50. doi:10.1111/j.1474-9726.2009.00467.x. PMC 2759984. PMID 19245677.
- de Hostos EL (2008). A brief history of the coronin family. Sub-Cellular Biochemistry. Subcellular Biochemistry. 48. Madame Curie Bioscience Database. pp. 31–40. doi:10.1007/978-0-387-09595-0_4. ISBN 978-0-387-09594-3. PMID 18925369.
- Rybakin V, Clemen CS, Eichinger L (2008). The coronin family of proteins (first ed.). New York, N.Y.: Springer. pp. 1–5. ISBN 978-0387095943.
- Pieters J (2000). Coronin 1 in innate immunity. Sub-Cellular Biochemistry. Subcellular Biochemistry. 48. pp. 116–23. doi:10.1007/978-0-387-09595-0_11. ISBN 978-0-387-09594-3. PMID 18925376.
- Steinmetz MO, Jelesarov I, Matousek WM, Honnappa S, Jahnke W, Missimer JH, Frank S, Alexandrescu AT, Kammerer RA (Apr 2007). "Molecular basis of coiled-coil formation". Proceedings of the National Academy of Sciences of the United States of America. 104 (17): 7062–7. doi:10.1073/pnas.0700321104. PMC 1855353. PMID 17438295.
- McArdle B, Hofmann A (2000). Coronin structure and implications. Sub-Cellular Biochemistry. Subcellular Biochemistry. 48. pp. 56–71. doi:10.1007/978-0-387-09595-0_6. ISBN 978-0-387-09594-3. PMID 18925371.
External links
Further reading
- Rasmussen HH, van Damme J, Puype M, Gesser B, Celis JE, Vandekerckhove J (Dec 1992). "Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes". Electrophoresis. 13 (12): 960–9. doi:10.1002/elps.11501301199. PMID 1286667.
- Suzuki K, Nishihata J, Arai Y, Honma N, Yamamoto K, Irimura T, Toyoshima S (May 1995). "Molecular cloning of a novel actin-binding protein, p57, with a WD repeat and a leucine zipper motif". FEBS Letters. 364 (3): 283–8. doi:10.1016/0014-5793(95)00393-N. PMID 7758584.
- Grogan A, Reeves E, Keep N, Wientjes F, Totty NF, Burlingame AL, Hsuan JJ, Segal AW (Dec 1997). "Cytosolic phox proteins interact with and regulate the assembly of coronin in neutrophils". Journal of Cell Science. 110. 110 (24): 3071–81. PMID 9365277.
- Ferrari G, Langen H, Naito M, Pieters J (May 1999). "A coat protein on phagosomes involved in the intracellular survival of mycobacteria". Cell. 97 (4): 435–47. doi:10.1016/S0092-8674(00)80754-0. PMID 10338208.
- Vanguri VK, Wang S, Godyna S, Ranganathan S, Liau G (Apr 2000). "Thrombospondin-1 binds to polyhistidine with high affinity and specificity". The Biochemical Journal. 347 (Pt 2): 469–73. doi:10.1042/0264-6021:3470469. PMC 1220979. PMID 10749676.
- Oku T, Itoh S, Okano M, Suzuki A, Suzuki K, Nakajin S, Tsuji T, Nauseef WM, Toyoshima S (Apr 2003). "Two regions responsible for the actin binding of p57, a mammalian coronin family actin-binding protein". Biological & Pharmaceutical Bulletin. 26 (4): 409–16. doi:10.1248/bpb.26.409. PMID 12673016.
- Fu GK, Wang JT, Yang J, Au-Young J, Stuve LL (Jul 2004). "Circular rapid amplification of cDNA ends for high-throughput extension cloning of partial genes". Genomics. 84 (1): 205–10. doi:10.1016/j.ygeno.2004.01.011. PMID 15203218.
- Oku T, Itoh S, Ishii R, Suzuki K, Nauseef WM, Toyoshima S, Tsuji T (Apr 2005). "Homotypic dimerization of the actin-binding protein p57/coronin-1 mediated by a leucine zipper motif in the C-terminal region". The Biochemical Journal. 387 (Pt 2): 325–31. doi:10.1042/BJ20041020. PMC 1134960. PMID 15601263.
- Gatfield J, Albrecht I, Zanolari B, Steinmetz MO, Pieters J (Jun 2005). "Association of the leukocyte plasma membrane with the actin cytoskeleton through coiled coil-mediated trimeric coronin 1 molecules". Molecular Biology of the Cell. 16 (6): 2786–98. doi:10.1091/mbc.E05-01-0042. PMC 1142424. PMID 15800061.
- Anand PK, Kaul D (Sep 2005). "Downregulation of TACO gene transcription restricts mycobacterial entry/survival within human macrophages". FEMS Microbiology Letters. 250 (1): 137–44. doi:10.1016/j.femsle.2005.06.056. PMID 16040207.
- Liu CZ, Chen Y, Sui SF (Jan 2006). "The identification of a new actin-binding region in p57". Cell Research. 16 (1): 106–12. doi:10.1038/sj.cr.7310014. PMID 16467882.
- Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- Yan M, Di Ciano-Oliveira C, Grinstein S, Trimble WS (May 2007). "Coronin function is required for chemotaxis and phagocytosis in human neutrophils". Journal of Immunology. 178 (9): 5769–78. doi:10.4049/jimmunol.178.9.5769. PMID 17442961.