Constitutive heterochromatin
Constitutive heterochromatin domains are regions of DNA found throughout the chromosomes of eukaryotes.[2] The majority of constitutive heterochromatin is found at the pericentromeric regions of chromosomes, but is also found at the telomeres and throughout the chromosomes.[2] In humans there is significantly more constitutive heterochromatin found on chromosomes 1, 9, 16, 19 and Y.[3] Constitutive heterochromatin is composed mainly of high copy number tandem repeats known as satellite repeats, minisatellite and microsatellite repeats, and transposon repeats. In humans these regions account for about 200Mb or 6.5% of the total human genome, but their repeat composition makes them difficult to sequence, so only small regions have been sequenced.

Visualization of constitutive heterochromatin is possible by using the C-banding technique. The regions that stain darker are regions of constitutive heterochromatin.[4] The constitutive heterochromatin stains darker because of the highly condensed nature of the DNA.
Constitutive heterochromatin is not to be confused with facultative heterochromatin, which is less condensed, less stable, and much less polymorphic, and which does not stain when using the C-banding technique.
Function
Constitutive heterochromatin is found more commonly in the periphery of the nucleus attached to the nuclear membrane. This concentrates the euchromatic DNA in the center of the nucleus where it can be actively transcribed. During mitosis it is believed that constitutive heterochromatin is necessary for proper segregation of sister chromatids and centromere function.[6] The repeat sequences found at the pericentromeres are not conserved throughout many species and depend more on epigenetic modifications for regulation, while telomeres show more conserved sequences.[2]
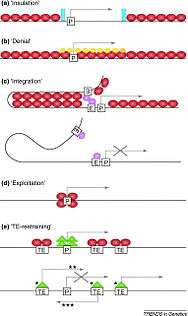
Constitutive heterochromatin was thought to be relatively devoid of genes, but researchers have found more than 450 genes in the heterochromatic DNA of Drosophila melanogaster.[5] These regions are highly condensed and epigenetically modified to prevent transcription. For the genes to be transcribed, they must have a mechanism to overcome the silencing that occurs in the rest of the heterochromatin. There are many proposed models for how the genes in these regions are expressed, including the insulation, denial, integration, exploitation, and TE restraining models.
When genes are placed near a region of constitutive heterochromatin, their transcription is usually silenced. This is known as position-effect variegation and can lead to a mosaic phenotype.
Replication and epigenetics
Constitutive heterochromatin is replicated late in S phase of the cell cycle and does not participate in meiotic recombination.
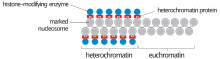
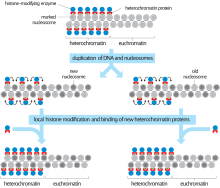
Histone modifications are one of the main ways that the cell condenses constitutive heterochromatin.[7] The three most common modifications in constitutive heterochromatin are histone hypoacetylation, histone H3-Lys9 methylation (H3K9), and cytosine methylation. These modifications are also found in other types of DNA, but much less frequently. Cytosine methylation is the most common type, although it is not found in all eukaryotes. In humans there is increased methylation at the centromeres and telomeres, which are composed of constitutive heterochromatin. These modifications can persist through both mitosis and meiosis and are heritable.
SUV39H1 is a histone methyltransferase that methylates H3K9, providing a binding site for heterochromatin protein 1 (HP1). HP1 is involved in the chromatin condensing process that makes DNA inaccessible for transcription.[8][9]
Diseases
Genetic disorders that result from mutations involving the constitutive heterochromatin tend to affect cell differentiation and are inherited in an autosomal recessive pattern.[6] Disorders include Roberts syndrome and ICF syndrome.
Some cancers are associated with anomalies in constitutive heterochromatin and the proteins involved in its formation and maintenance. Breast cancer is linked to a decrease in the HP1 alpha protein, while non-Hodgkin's lymphoma is linked to hypomethylation of the genome and especially of satellite regions.
References
- "C-Banding". web.udl.es. Retrieved 2015-12-02.
- Saksouk, Nehmé; Simboeck, Elisabeth; Déjardin, Jérôme (2015-01-15). "Constitutive heterochromatin formation and transcription in mammals". Epigenetics & Chromatin. 8: 3. doi:10.1186/1756-8935-8-3. ISSN 1756-8935. PMC 4363358. PMID 25788984.
- T. Strachan and A. Read (2004). Human Molecular Genetics 3. Garland Publishing. pp. 256–295. ISBN 978-0-81534182-6.
- Angell, Roslyn R.; Jacobs, Patricia A. (1975-12-01). "Lateral asymmetry in human constitutive heterochromatin". Chromosoma. 51 (4): 301–310. doi:10.1007/BF00326317. ISSN 0009-5915.
- Yasuhara, Jiro C.; Wakimoto, Barbara T. (2006-06-01). "Oxymoron no more: the expanding world of heterochromatic genes". Trends in Genetics. 22 (6): 330–338. doi:10.1016/j.tig.2006.04.008. PMID 16690158.
- Marie-Geneviève Mattei and Judith Luciani. "Heterochromatin, from Chromosome to Protein". Atlas of Genetics and Cytogenetics in Oncology and Haematology. Archived from the original on 27 October 2015. Retrieved 9 November 2015.
- Richards, Eric J.; Elgin, Sarah C. R. (2002). "Epigenetic Codes for Heterochromatin Formation and Silencing". Cell. 108 (4): 489–500. doi:10.1016/S0092-8674(02)00644-X. ISSN 0092-8674. PMID 11909520.
- Bártová, Eva; Krejčí, Jana; Harničarová, Andrea; Galiová, Gabriela; Kozubek, Stanislav (2008-08-01). "Histone Modifications and Nuclear Architecture: A Review". Journal of Histochemistry & Cytochemistry. 56 (8): 711–721. doi:10.1369/jhc.2008.951251. ISSN 0022-1554. PMC 2443610. PMID 18474937.
- Lomberk, Gwen; Wallrath, Lori; Urrutia, Raul (2006-01-01). "The Heterochromatin Protein 1 family". Genome Biology. 7 (7): 228. doi:10.1186/gb-2006-7-7-228. ISSN 1465-6906. PMC 1779566. PMID 17224041.