Collins reagent
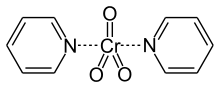
Collins reagent is the complex of chromium(VI) oxide with pyridine in dichloromethane.[2] This metal-pyridine complex, a red solid, is used to oxidize primary alcohols to the aldehyde. This complex is a hygroscopic orange solid.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Pyridine - trioxochromium (2:1) | |
| Other names
Dipyridine chromium(VI) oxide[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C10H10CrN2O3 | |
| Molar mass | 258.194 |
| Appearance | Red crystals[1] |
| Density | 1.565 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and structure
The complex is produced by treating chromium trioxide with pyridine.[2] The complex is diamagnetic. According to X-ray crystallography, the complex is 5-coordinate with mutually trans pyridine ligands. The Cr-O and Cr-N distances are respectively 163 and 215 picometers.[3]
In terms of history, the complex was first produced by Sisler et al.[4]
Reactions
Collins reagent is especially useful for oxidations of acid sensitive compounds. Primary and secondary alcohols are oxidized respectively to aldehydes and ketones in yields of 87-98%.[5]
Like other oxidations by Cr(VI), the stoichiometry of the oxidations is complex because the metal undergoes 3e reduction and the substrate is oxidized by 2 electrons:
- 3 RCH2OH + 2 CrO3(pyridine)2 → 3 RCHO + 3 H2O + Cr2O3 + 4 pyridine
The reagent is typically used in a sixfold excess. Methylene chloride is the typical solvent, with the solubility of 12.5 g/100 mL.
The application of this reagent to oxidations was discovered by G. I. Poos, G. E. Arth, R. E. Beyler and L.H. Sarett in 1953. It was popularized by Collins several years later.[6]
Other reagents
- Sarett oxidation
- Oxidation with chromium(VI)-amine complexes
Collins reagent can be used as an alternative to the Jones reagent and pyridinium chlorochromate (PCC) when oxidizing secondary alcohols to ketones. PCC and pyridinium dichromate (PDC) oxidations have largely supplanted Collins oxidation.[1]
Safety and environmental aspects
The solid is flamable.[1] Generally speaking chromium(VI) compounds are carcinogenic.
References
- Fillmore Freeman (2001). "Dipyridine Chromium(VI) Oxide". Encyclopedia of Reagents for Organic Synthesis. e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd452m. ISBN 0471936235.
- J. C. Collins, W.W. Hess (1972). "Aldehydes from Primary Alcohols by Oxidation with Chromium Trioxide: Heptanal". 52: 5. doi:10.15227/orgsyn.052.0005. Cite journal requires
|journal=(help) - Cameron, T Stanley; Clyburne, Jason AC; Dubey, Pramod K.; Grossert, J Stuart; Ramaiah, K.; Ramanatham, J.; Sereda, Sergei V. (2003). "Compounds of chromium(VI): The Pyridine.Chromic Anhydride Complex, Benzimidazolinium Dichromate, and Three 2-Alkyl-1H-benzimidazolinium Dichromates". Canadian Journal of Chemistry. 81 (6): 612–619. doi:10.1139/v03-042.
- Sisler, Harry H.; Bush, Jack D.; Accountius, Oliver E. (1948). "Addition Compounds of Chromic Anhydride with Some Heterocyclic Nitrogen Bases". Journal of the American Chemical Society. 70 (11): 3827–3830. doi:10.1021/ja01191a085. PMID 18102959.
- Ronald Ratcliffe and Ronald Rodehorst (1970). "Improved Procedure for Oxidations with the Chromium Trioxide-Pyridine Complex". J. Org. Chem. 35 (11): 4000–4001. doi:10.1021/jo00836a108.
- J. C. Collins, W. W. Hess and F. J. Frank (1968). "Dipyridine-chromium(VI) oxide oxidation of alcohols in dichloromethane". Tetrahedron Lett. 9 (30): 3363–3366. doi:10.1016/S0040-4039(00)89494-0.