Collins oxidation
The Collins oxidation is an organic reaction for the oxidation of primary alcohols to aldehydes. It is distinguished from other chromium oxide-based oxidations by the use of Collins reagent, a complex of chromium(VI) oxide with pyridine in dichloromethane.[1][2]
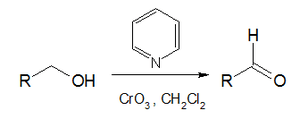
| Collins oxidation | |
|---|---|
| Named after | Joseph C. Collins |
| Reaction type | Organic redox reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000550 |
Related oxidation reactions
Several chromium oxides are used for related oxidations.[3] These include Jones oxidation and Sarett oxidation.
gollark: It's data from phones running, presumably, Google spying software, which mine is not because it runs a custom ROM without that.
gollark: See, this is why I try to avoid running Google software on my device.
gollark: Apparently it has an effect on viral replication somehow.
gollark: As far as I know he isn't actually *in* China, just... talks about it sometimes?
gollark: Has the link and elected to join it, that is.
See also
References
- J. C. Collins, W. W. Hess and F. J. Frank (1968). "Dipyridine-chromium(VI) oxide oxidation of alcohols in dichloromethane". Tetrahedron Lett. 9 (30): 3363–3366. doi:10.1016/S0040-4039(00)89494-0.
- J. C. Collins, W.W. Hess (1988). "Aldehydes from Primary Alcohols by Oxidation with Chromium Trioxide: Heptanal". Organic Syntheses.; Collective Volume, 6, p. 644
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.