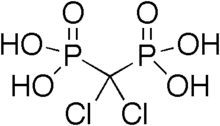
Clodronic acid
Clodronic acid (INN) or clodronate disodium (USAN) is a first generation (non-nitrogenous) bisphosphonate. It is an anti-osteoporotic drug approved for the prevention and treatment of osteoporosis in post-menopausal women and men to reduce vertebral fractures, hyperparathyroidism, hypercalcemia in malignancy, multiple myeloma and fracture related pain because of its potent anti-inflammatory and analgesic effects shown as a reduction in inflammatory markers like IL-1β, IL-6, and TNF-α.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.031.090 |
| Chemical and physical data | |
| Formula | CH4Cl2O6P2 |
| Molar mass | 244.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Use in human medicine
An Italian study compared the analgesic effect of clodronic acid versus acetaminophen in rheumatic condition related pain. Study result show a reduction in pain in favor of clodronic acid that provided more analgesia than 3 grams/day of acetaminophen. Clodronic acid is also used in experimental medicine to selectively deplete macrophages.
Clodronic acid is approved for human use in Canada and Australia, the United Kingdom, where it is marketed as Bonefos, Loron, Clodron and in Italy as Clasteon, Difosfonal, Osteostab and several generics. In other countries is prescribed as a bone resorption inhibitor and antihypercalcemic agent. It is not approved for use in the United States, because it has too many adverse effects.
Use in equine medicine
Clodronic acid is approved for use in horses under the trade name Osphos, for treatment of bone resorptive processes of navicular syndrome. It is given by intramuscular injection at one point in time, with the total dose divided into 2-3 sites on the horse. Clinical effects (e.g. improvement of lameness) after a single treatment can be seen up to 6 months post-treatment.
Notes and references
- Pennanen, Niina; Lapinjoki, Seppo; Urtti, Arto; Mönkkönen, Jukka (1995). "Effect of Liposomal and Free Bisphosphonates on the IL-1β, IL-6 and TNFα Secretion from RAW 264 Cells in Vitro". Pharmaceutical Research. 12 (6): 916–922. doi:10.1023/A:1016281608773. ISSN 0724-8741.
- U.S. Food and Drug Administration. "FDA Provides Equine Veterinarians with Important Information about TILDREN and OSPHOS for Navicular Syndrome in Horses". Retrieved 3 January 2015.