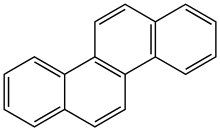
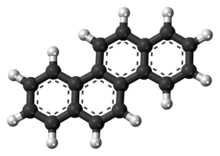
Chrysene
Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12[3] that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5-6 mg/kg.[4]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Chrysene | |
| Other names
1,2-Benzophenanthrene; 1,2-Benzphenanthrene; Benzo[a]phenanthrene; NSC 6175; [4]Phenacene | |
| Identifiers | |
3D model (JSmol) |
|
| 1909297 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.386 |
| EC Number |
|
| 262600 | |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H12 | |
| Molar mass | 228.294 g·mol−1 |
| Appearance | white solid |
| Density | 1.274 g/cm3 |
| Melting point | 254 °C (489 °F; 527 K) |
| Boiling point | 448 °C (838 °F; 721 K) |
| Insoluble | |
| Solubility in ethanol | 1 g/1300 mL[2] |
| -166.67·10−6 cm3/mol | |
| Related compounds | |
Related PAHs |
Pyrene, Tetracene, Triphenylene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The name "chrysene" originates from Greek Χρύσoς (chrysos), meaning "gold", and is due to the golden-yellow color of the crystals of the hydrocarbon, thought to be the proper color of the compound at the time of its isolation and characterization. However, high purity chrysene is colorless, the yellow hue being due to the traces of its yellow-orange isomer tetracene, which cannot be separated easily.
Occurrence
Chrysene is a constituent of tobacco smoke.[5]
Safety
As with other PAHs, chrysene is suspected to be a human carcinogen. Some evidence suggests that it causes cancer in laboratory animals,[6] but chrysene is often contaminated with more strongly carcinogenic compounds. Chrysene is estimated to have about 1% of the toxicity of benzo(a)pyrene.[7]
Derivatives
Derivatives of chrysene include tetrahydrochrysene and 2,8-dihydroxyhexahydrochrysene, which are estrogenic compounds. The experimental cancer drug crisnatol is a derivative of chrysene.
See also
References
- Merck Index, 11th Edition, 2259.
- Merck Index, 14th edition
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 6 (11th ed.). Cambridge University Press. p. 319.
- Anja Sörensen and Bodo Wichert "Asphalt and Bitumen" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2009. doi:10.1002/14356007.a03_169.pub2http://www.qrpoil.com/site/?bitumen Archived 2016-03-04 at the Wayback Machine
- Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). "Hazardous Compounds in Tobacco Smoke". International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
- TOXICOLOGICAL PROFILE FOR POLYCYCLIC AROMATIC HYDROCARBONS
- Ian C.T. Nisbet, Peter K. LaGoy "Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs)", Regulatory Toxicology and Pharmacology 1992, Volume 16, Pages 290-300. doi:10.1016/0273-2300(92)90009-X