Clofedanol
Clofedanol (INN) or chlophedianol (BAN) is a centrally acting cough suppressant used in the treatment of dry cough. Clofedanol has local anesthetic and antihistamine properties, and may have anticholinergic effects at high doses.[1] It is marketed in Canada under the trade name Ulone.GM Pharmaceuticals owns the patents to Chlophedianol and was the first company to launch the cough suppressant in the United States archive-date = 2009-08-21 | url-status = dead }}</ref> Chlophedianol was approved for OTC status in 1987 by the FDA OTC monograph process[2] and its safety and efficacy data are limited.
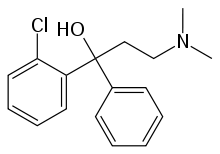 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.219 |
| Chemical and physical data | |
| Formula | C17H20ClNO |
| Molar mass | 289.80 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Synthesis
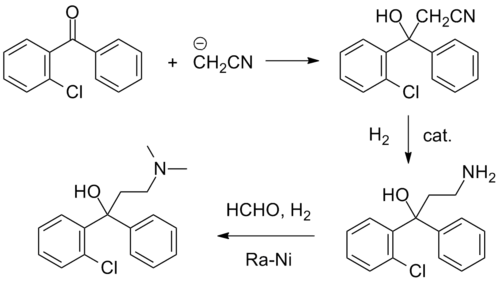
Clofedanol synthesis: R. Lorenz and H. Henecka, U.S. Patent 3,031,377 (1962).
gollark: Plus the fact that TJ09 ignores 90% of things then just makes some slightly relevant comment on a random thread somewhere.
gollark: Oh, plus it being significantly easier to just say "use wants as haves" instead of adding a have box.
gollark: It took lots of people complaining and several weeks to change the rules of the hub to something remotely sane, and they're still broken and you don't even get told why you're banned.
gollark: I suspect it was just accidentally mistyping a number.
gollark: I'd also like to note the fact that the original trade hub was the minimum viable product and didn't even have proper rules.
See also
References
- "Clofedanol" (in French). BIAM. 1998-07-24. Retrieved 2007-04-15.
- "Department of Health and Human Services. Food and Drug Administration. 21 CFR Parts 310, 341, and 369. Docket No. 76N-052T. Cold, cough, allergy, bronchodilator, and antiasthmatic drug products for over-the0counter human use; final monograph for OTC antitussive drug products. Federal Register 1987;52(155):30042-57" (PDF). FDA.gov. 1987-08-12.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.