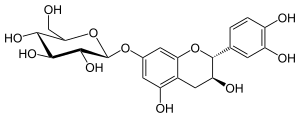
Catechin-7-O-glucoside
Catechin-7-O-glucoside is a flavan-3-ol glycoside formed from catechin.
 | |
| Names | |
|---|---|
| IUPAC name
(2S,4S,5S)-2-[[(2R,3S)-2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-3,4-dihydro-2H-chromen-7-yl]oxy]-6-(hydroxymethyl)oxane-3,4,5-triol | |
| Other names
(2R,3S)-Catechin-7-O-β-D-glucopyranoside Catechin 7-O-β-glucopyranoside (+)-Catechin 7-O-β-glucoside (+)-Catechin 7-O-beta-D-glucopyranoside Catechin 7-glucoside C7G CA-G | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H24O11 | |
| Molar mass | 452.412 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Natural occurrences
Catechin-7-O-glucoside can be isolated from the hemolymph of the European pine sawfly (Neodiprion sertifer).[1] It also occurs in relatively large quantities in cowpea (Vigna unguiculata) as the dominant flavan-3-ol monomer, and actually accounts for up to 70% of cowpea proanthocyanidins (tannins).[2]
It can also be produced by biotransformation of (+)-catechin by cultured cells of Eucalyptus perriniana.[3]
Presence in natural traditional drugs
Catechin-7-O-glucoside can be found in Paeoniae Radix, the crude drug made from roots of the Chinese peony (Paeonia lactiflora),[4] in the red knotweed (Bistorta macrophylla, also known as Polygonum macrophyllum),[5] in the stem barks of the Nepali hog plum (Choerospondias axillaris),[6] in the Korean plum yew (Cephalotaxus koreana)[7] and in Huanarpo Macho (Jatropha macrantha).[8] (−)-Catechin 7-O-β-d-glucopyranoside is found in the bark of Rhaphiolepis umbellata.[9]
Health effects
This compound has an antioxidant activity leading to a cytoprotective effect.[11][13]
References
- Vihakas, Matti; Tähtinen, Petri; Ossipov, Vladimir; Salminen, Juha-Pekka (2012). "Flavonoid Metabolites in the Hemolymph of European Pine Sawfly (Neodiprion sertifer) Larvae". Journal of Chemical Ecology. 38 (5): 538–46. doi:10.1007/s10886-012-0113-y. PMID 22527054.
- Ojwang, Leonnard O.; Yang, Liyi; Dykes, Linda; Awika, Joseph (2013-08-15). "Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O-glucoside as the dominant compound". Food Chemistry. 139 (1–4): 35–43. doi:10.1016/j.foodchem.2013.01.117. PMID 23561075.
- Biotransformation of (+)-catechin by plant cultured cells of Eucalyptus perriniana. Otani S, Kondo Y, Asada Y, Furuya, Hamada, Nakajima, Ishihara and Hamada H, Plant Biotechnol., 2004, Vol. 21, No. 5, pages 407-409 (abstract Archived 2009-07-22 at the Wayback Machine)
- New Monoterpene Glycoside Esters and Phenolic Constituents of Paeoniae Radix, and Increase of Water Solubility of Proanthocyanidins in the Presence of Paeoniflorin. Takashi Tanaka, Maki Kataoka, Nagisa Tsuboi and Isao Kouno, Chem. Pharm. Bull., 2000, 48(2), pages 201—207
- Wang, S; Wang, D; Feng, S (2004). "Studies on chemical constituents from Polygonum macrophyllum". Journal of Chinese Medicinal Materials. 27 (6): 411–3. PMID 15524292.
- Flavanoidal constituents of Choerospondias axillaries and their in vitro antitumor and anti-hypoxia activities. Li Chang-wei, Cui Cheng-bin, Cai Bing, Han Bing, Li Ming-ming and Fan Ming, Chinese Journal of Medicinal Chemistry, 2009, 19 (1), pages 48-51,64 (abstract Archived 2014-03-09 at the Wayback Machine)
- Yoon, Kee Dong; Jeong, Doc Gyun; Hwang, Yun Ha; Ryu, Jei Man; Kim, Jinwoong (2007). "Inhibitors of Osteoclast Differentiation fromCephalotaxus koreana". Journal of Natural Products. 70 (12): 2029–32. doi:10.1021/np070327e. PMID 17994703.
- Benavides, Angelyne; Montoro, Paola; Bassarello, Carla; Piacente, Sonia; Pizza, Cosimo (2006). "Catechin derivatives in Jatropha macrantha stems: Characterisation and LC/ESI/MS/MS quali–quantitative analysis". Journal of Pharmaceutical and Biomedical Analysis. 40 (3): 639–47. doi:10.1016/j.jpba.2005.10.004. PMID 16300918.
- Flavanol glucosides from rhubarb and Rhaphiolepis umbellata. Gen-Ichiro Nonaka, Emiko Ezakia, Katsuya Hayashia and Itsuo Nishioka, Phytochemistry, Volume 22, Issue 7, 1983, Pages 1659–1661, doi:10.1016/0031-9422(83)80105-8
- Report on cereals at Phenol-Explorer.eu. Retrieved 18 December 2012.
- Baek, Jin-A; Son, Young-Ok; Fang, Minghao; Lee, Young Jae; Cho, Hyoung-Kwon; Whang, Wan Kyunn; Lee, Jeong-Chae (2011). "Catechin-7-O-β-d-glucopyranoside scavenges free radicals and protects human B lymphoma BJAB cells on H2O2-mediated oxidative stress". Food Science and Biotechnology. 20: 151–158. doi:10.1007/s10068-011-0021-x., INIST:23809899
- Friedrich, Wolfgang; Galensa, Rudolf (2002). "Identification of a new flavanol glucoside from barley ( Hordeum vulgare L.) and malt". European Food Research and Technology. 214 (5): 388. doi:10.1007/s00217-002-0498-x.
- Kim, Ki Cheon; Kim, Jin Sook; Ah Kang, Kyoung; Kim, Jong Min; Won Hyun, Jin (2010). "Cytoprotective effects of catechin 7-O-β-D glucopyranoside against mitochondrial dysfunction damaged by streptozotocin in RINm5F cells". Cell Biochemistry and Function. 28 (8): 651–60. doi:10.1002/cbf.1703. PMID 21104932.