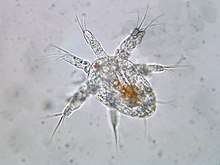
Crustacean larva
Crustaceans may pass through a number of larval and immature stages between hatching from their eggs and reaching their adult form. Each of the stages is separated by a moult, in which the hard exoskeleton is shed to allow the animal to grow. The larvae of crustaceans often bear little resemblance to the adult, and there are still cases where it is not known what larvae will grow into what adults. This is especially true of crustaceans which live as benthic adults (on the sea bed), more-so than where the larvae are planktonic, and thereby easily caught.
Many crustacean larvae were not immediately recognised as larvae when they were discovered, and were described as new genera and species. The names of these genera have become generalised to cover specific larval stages across wide groups of crustaceans, such as zoea and nauplius. Other terms described forms which are only found in particular groups, such as the glaucothoe of hermit crabs, or the phyllosoma of slipper lobsters and spiny lobsters.
Life cycle
At its most complete, a crustacean's life cycle begins with an egg, which is usually fertilised, but may instead be produced by parthenogenesis. This egg hatches into a pre-larva or pre-zoea. Through a series of moults, the young animal then passes through various zoea stages, followed by a megalopa or post-larva. This is followed by metamorphosis into an immature form, which broadly resembles the adult, and after further moults, the adult form is finally reached. Some crustaceans continue to moult as adults, while for others, the development of gonads signals the final moult.
Any organs which are absent from the adults do not generally appear in the larvae, although there are a few exceptions, such as the vestige of the fourth pereiopod in the larvae of Lucifer, and some pleopods in certain Anomura and crabs.[1] In a more extreme example, the Sacculina and other Rhizocephala have a distinctive nauplius larva with its complex body structure, but the adult form lacks many organs due to extreme adaptation to its parasitic life style.
History of the study of crustacean larva
Antonie van Leeuwenhoek was the first person to observe the difference between larval crustaceans and the adults when he watched the eggs of Cyclops hatching in 1699.[1] Despite this, and other observations over the following decades, there was controversy among scientists about whether or not metamorphosis occurred in crustaceans, with conflicting observations presented, based on different species, some of which went through a metamorphosis, and some of which did not. This controversy persisted until the 1840s, and the first descriptions of a complete series of larval forms were not published until the 1870s (Sidney Irving Smith on the American lobster in 1873; Georg Ossian Sars on the European lobster in 1875, and Walter Faxon on the shrimp Palaemonetes vulgaris in 1879).[1]
Larval stages

Nauplius
The genus name Nauplius was published posthumously by Otto Friedrich Müller in 1785 for animals now known to be the larvae of copepods. The nauplius stage (plural: nauplii) is characterised by the use of the appendages of the head (the antennae) for swimming. The nauplius is also the stage at which a simple, unpaired eye first appears. The eye is known for that reason as the "naupliar eye", and is often absent in later developmental stages, although it is retained into the adult form in some groups, such as the Notostraca.
Zoea
The genus Zoea was initially described by Louis Augustin Guillaume Bosc in 1802 for an animal now known to be the larva of a crab.[1] The zoea stage (plural: zoeas or zoeae) is characterised by the use of the thoracic appendages for swimming and a large dorsal spine.
Post-larva
The post-larva is characterised by the use of abdominal appendages (pleopods) for propulsion. The post-larva is usually similar to the adult form, and so many names have been erected for the stage in different groups. William Elford Leach erected the genus Megalopa in 1813 for a post-larval crab; a copepod post-larva is called a copepodite; a barnacle post larva is called a cypris; a shrimp post-larva is called a parva; an hermit crab post-larva are called glaucothoe; spiny lobster / furry lobsters post-larva is called a puerulus and a slipper lobster post-larva is called a nisto
Larvae of crustacean groups
Branchiopoda
In the Branchiopoda, the most basal group of crustaceans, there is no metamorphosis; instead, the animal grows through a series of moults, with each moult adding various numbers of segments to the body, but without any dramatic changes in form. Every other crustacean group with free larvae shows a metamorphosis, and this difference in the larvae is thought to reflect "a fundamental cleavage" of the crustaceans.[1]
Cephalocarida
In the Mediterranean horseshoe shrimp Lightiella magdalenina, the young experience 15 stages following the nauplius, termed metanaupliar stages, and two juvenile stages, with each of the first six stages adding two trunk segments, and the last four segments being added singly.[2]
Remipedia
The larvae of remipedes are lecithotrophic, consuming egg yolk rather than using external food sources. This characteristic, which is shared with malacostracan groups such as the Decapoda and Euphausiacea (krill) has been used to suggest a link between Remipedia and Malacostraca.[3]
Malacostraca
Amphipod hatchlings resemble the adults.[4]
Young isopod crustaceans hatch directly into a manca stage, which is similar in appearance to the adult. The lack of a free-swimming larval form has led to high rates of endemism in isopods, but has also allowed them to colonise the land, in the form of the woodlice.
Stomatopoda
The larvae of many groups of mantis shrimp are poorly known. In the superfamily Lysiosquilloidea, the larvae hatch as antizoea larvae, with five pairs of thoracic appendages, and develop into erichthus larvae, where the pleopods appear. In the Squilloidea, a pseudozoea larva develops into an alima larva, while in Gonodactyloidea, a pseudozoea develops into an erichthus.[5]
A single fossil stomatopod larva has been discovered, in the Upper Jurassic Solnhofen lithographic limestone.[6]

Krill
The life cycle of krill is relatively well understood, although there are minor variations in detail from species to species. After hatching, the larvae go through several stages called nauplius, pseudometanauplius, metanauplius, calyptopsis and furcilia stages, each of which is sub-divided into several sub-stages. The pseudometanauplius stage is exclusive to the so-called "sac-spawners". Until the metanauplius stage, the larvae are reliant on the yolk reserves, but from the calyptopsis stage, they begin to feed on phytoplankton. During the furcilia stages, segments with pairs of swimmerets are added, beginning at the frontmost segments, with each new pair only becoming functional at the next moult. After the final furcilia stage, the krill resembles the adult.

Decapoda

Apart from the prawns of the suborder Dendrobranchiata, all decapod crustaceans brood their eggs on the female's pleopods. This has resulted in development in decapod crustaceans being generally abbreviated.[1] There are at most nine larval stages in decapods, as in krill, and both decapod nauplii and krill nauplii often lack mouthparts and survive on nutrients supplied in the egg yolk (lecithotrophy). In species with normal development, eggs are roughly 1% of the size of the adult; in species with abbreviated development, and therefore more yolk in the eggs, the eggs may reach 1/9 of the adult's size.[1]
The post-larva of shrimp is called parva, after the species Acanthephyra parva described by Henri Coutière, but which was later recognised as the larva of Acanthephyra purpurea.[7]
In the marine lobsters, there are three larval stages, all similar in appearance.
Freshwater crayfish embryos differ from those of other crustaceans in having 40 ectoteloblast cells, rather than around 19.[8] The larvae show abbreviated development, and hatch with a full complement of adult appendages with the exceptions of the uropods and the first pair of pleopods.[1]

The larvae of the Achelata (slipper lobsters, spiny lobsters and furry lobsters) are unlike any other crustacean larvae. The larvae are known as phyllosoma, after the genus Phyllosoma erected by William Elford Leach in 1817. They are flattened and transparent, with long legs and eyes on long eyestalks. After passing through 8–10 phyllosoma stages, the larva undergoes "the most profound transformation at a single moult in the Decapoda", when it develops into the so-called puerulus stage, which is an immature form resembling the adult animal.[1]
The members of the traditional infraorder Thalassinidea can be divided into two groups on the basis of their larvae. According to Robert Gurney,[1] the "homarine group" comprises the families Axiidae and Callianassidae, while the "anomuran group" comprises the families Laomediidae and Upogebiidae. This split corresponds with the division later confirmed with molecular phylogenetics.[9]
Among the Anomura, there is considerable variation in the number of larval stages. In the South American freshwater genus Aegla, the young hatch from the eggs in the adult form.[1] Squat lobsters pass through four, or occasionally five, larval states, which have a long rostrum, and a spine on either side of the carapace; the first post-larva closely resembles the adult.[1] Porcelain crabs have two or three larval stages, in which the rostrum and the posterior spine on the carapace are "enormously long".[1] Hermit crabs pass through around four larval stages. The post-larva is known as the glaucothoe, after a genus named by Henri Milne-Edwards in 1830.[1] The glaucothoe is 3 millimetres (0.12 in) long in Pagurus longicarpus, but glaucothoe larvae up to 20 mm (0.79 in) are known, and were once thought to represent animals which had failed to develop correctly.[1] Like the preceding stages, the glaucothoe is symmetrical, and although the glaucothoe begins as a free-swimming form, it often acquires a gastropod shell to live in; the coconut crab, Birgus latro, always carries a shell when the immature animal comes ashore, but this is discarded later.[1]


Although they are classified as crabs, the larvae of Dromiacea are similar to those of the Anomura, which led many scientists to place dromiacean crabs in the Anomura, rather than with the other crabs. Apart from the Dromiacea, all crabs share a similar and distinctive larval form. The crab zoea has a slender, curved abdomen and a forked telson, but its most striking features are the long rostral and dorsal spines, sometimes augmented by further, lateral spines.[1] These spines can be many times longer than the body of the larva. Crab prezoea larvae have been found fossilised in the stomach contents of the Early Cretaceous bony fish Tharrhias.[10]
Maxillopoda
Copepoda
Copepods have six naupliar stages, followed by a stage called the copepodid, which has the same number of body segments and appendages in all copepods. The copepodid larva has two pairs of unsegmented swimming appendages, and an unsegmented "hind-body" comprising the thorax and the abdomen.[1] There are typically five copepodid stages, but parasitic copepods may stop after a single copepodid stage. Once the gonads develop, there are no further moults.[1]
Parasitic copepods
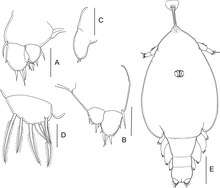
A, leg 3;
B, leg 3 (other specimen);
C, leg 4;
D, caudal ramus;
E, habitus of putative female, dorsal.
Scale bars: A–D = 0.025 mm; E = 0.2 mm.[11]
Chalimus (plural chalimi) is a stage of development of a copepod parasite of fish, such as the salmon louse (Lepeophtheirus salmonis).[12][13]
Chalimus Burmeister, 1834 is also a synonym for Lepeophtheirus Nordmann, 1832.
| Look up Chalimus in Wiktionary, the free dictionary. |
Facetotecta
The single genus in the Facetotecta, Hansenocaris, is only known from its larvae. They were first described by Christian Andreas Victor Hensen in 1887, and named "y-nauplia" by Hans Jacob Hansen, assuming them to be the larvae of barnacles.[14] The adults are presumed to be parasites of other animals.[15]
See also
References
- Robert Gurney (1942). Larvae of decapod crustacea (PDF). London: Ray Society. pp. 1–306.
- Alberto Addis; Francesca Biagi; Antonello Floris; Emiliana Puddu; Marcella Carcupino (2007). "Larval development of Lightiella magdalenina (Crustacea, Cephalocarida)". Marine Biology. 152 (3): 733–744. doi:10.1007/s00227-007-0735-8.
- Stefan Koenemann; Jørgen Olesen; Frederike Alwes; Thomas Iliffe; Mario Hoenemann; Petra Ungerer; Carsten Wolff; Gerhard Scholtz (2009). "The post-embryonic development of Remipedia (Crustacea)—additional results and new insights". Development Genes and Evolution. 219 (3): 131–145. doi:10.1007/s00427-009-0273-0. PMID 19184096.
- "The Biology of Amphipods". Museum Victoria. Archived from the original on September 18, 2010. Retrieved June 7, 2010.
- S. T. Ahyong; J. K. Lowry. "Stomatopoda: Families". World Crustacea. Australian Museum. Archived from the original on December 14, 2010. Retrieved June 6, 2010.
- Joachim T. Haug; Carolin Haug; Manfred Ehrlich (2008). "First fossil stomatopod larva (Arthropoda: Crustacea) and a new way of documenting Solnhofen fossils (Upper Jurassic, Southern Germany)" (PDF). Palaeodiversity. 1: 103–109.
- Kemp, Stanley W. (1907). "XI. Biscayan Plankton. Part XI.-Decapoda". Transactions of the Linnean Society of London. 2nd Series: Zoology. 10 (8): 205–217. doi:10.1111/j.1096-3642.1907.tb00072.x.
- G. Scholtz; S. Richter (1995). "Phylogenetic systematics of the reptantian Decapoda (Crustacea, Malacostraca)". Zoological Journal of the Linnean Society. 113 (3): 289–328. doi:10.1006/zjls.1995.0011.
- Sammy De Grave; N. Dean Pentcheff; Shane T. Ahyong; et al. (2009). "A classification of living and fossil genera of decapod crustaceans" (PDF). Raffles Bulletin of Zoology. Suppl. 21: 1–109. Archived from the original (PDF) on 2011-06-06.
- John G. Maisey & Maria da Gloria P. de Carvalho (1995). "First records of fossil sergestid decapods and fossil brachyuran crab larvae (Arthropoda, Crustacea), with remarks on some supposed palaemonid fossils, from the Santana Formation (Aptian-Albian, NE Brazil)" (PDF). American Museum Novitates. 3132: 1–20.
- Venmathi Maran, Balu Alagar; Moon, Seong Yong; Ohtsuka, Susumu; Oh, Sung-Yong; Soh, Ho Young; Myoung, Jung-Goo; Iglikowska, Anna; Boxshall, Geoffrey Allan (2013). "The caligid life cycle: new evidence from Lepeophtheirus elegans reconciles the cycles of Caligus and Lepeophtheirus (Copepoda: Caligidae)". Parasite. 20: 15. doi:10.1051/parasite/2013015. PMC 3718518. PMID 23647664.

- The salmon louse Lepeophtheirus salmonis (Copepoda: Caligidae) life cycle has only two chalimus stages. LA Hamre, C Eichner, CMA Caipang, ST Dalvin…, PLOS One, 2013
- Ultrastructure of the frontal filament in chalimus larvae of Caligus elongatus and Lepeophtheirus salmonis from Atlantic salmon, Salmo salar. AW Pike, K Mackenzie, A Rowand, Pathogens of wild and farmed fish: sea lice, 1993
- E. A. Ponomarenko (2006). "Facetotecta – unsolved riddle of marine biology". Russian Journal of Marine Biology. 32 (Suppl. 1): S1–S10. doi:10.1134/S1063074006070017.
- Gerhard Scholtz (2008). "Zoological detective stories: the case of the facetotectan crustacean life cycle". Journal of Biology. 7 (5): 16. doi:10.1186/jbiol77. PMC 2447532. PMID 18598383.