Dimethylsulfoniopropionate
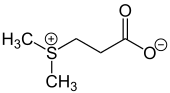
Dimethylsulfoniopropionate (DMSP), is an organosulfur compound with the formula (CH3)2S+CH2CH2COO−. This zwitterionic metabolite can be found in marine phytoplankton, seaweeds, and some species of terrestrial and aquatic vascular plants. It functions as an osmolyte as well as several other physiological and environmental roles have also been identified.[3] DMSP was first identified in the marine red alga Polysiphonia fastigiata.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-(dimethylsulfaniumyl)propanoate | |
| Other names
dimethyl-β-propiothetin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.228.826 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O2S | |
| Molar mass | 134.1967 |
| Appearance | white crystalline hygroscopic powder with a characteristic odor.[1] |
| Melting point | 120 to 125 °C (248 to 257 °F; 393 to 398 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
In higher plants, DMSP is biosynthesized from S-methylmethionine. Two intermediates in this conversion are dimethylsulfoniumpropylamine and dimethylsulfoniumpropionaldehyde.[5] In algae, however, the biosynthesis starts with the removal of the amino group from methionine, rather than from S-methylmethionine.[6]
Degradation
DMSP is broken down by marine microbes to form two major volatile sulfur products, each with distinct effects on the environment. One of its breakdown products is methanethiol (CH3SH), which is assimilated by bacteria into protein sulfur. Another volatile breakdown product is dimethyl sulfide (CH3SCH3; DMS). There is evidence that DMS in seawater can be produced by cleavage of dissolved (extracellular) DMSP[7][8] by the enzyme DMSP-lyase, although many non-marine species of bacteria convert methanethiol to DMS.
DMS is also taken up by marine bacteria, but not as rapidly as methanethiol. Although DMS usually consists of less than 25% of the volatile breakdown products of DMSP, the high reactivity of methanethiol makes the steady-state DMS concentrations in seawater approximately 10 times those of methanethiol (~3 nM vs. ~0.3 nM). Curiously, there have never been any published correlations between the concentrations of DMS and methanethiol. This is probably due to the non-linear abiotic and microbial uptake of methanethiol in seawater, and the comparatively low reactivity of DMS. However, a significant portion of DMS in seawater is oxidized to dimethyl sulfoxide (DMSO).
Relevant to global climate, DMS is thought to play a role in the Earth's heat budget by decreasing the amount of solar radiation that reaches the Earth's surface. This occurs through degradation of DMS in the atmosphere into hygroscopic compounds that condense water vapor leading to the formation of clouds.[9]
DMSP has also been implicated in influencing the taste and odour characteristics of various products. For example, although DMSP is odourless and tasteless, it is accumulated at high levels in some marine herbivores or filter feeders. Increased growth rates, vigour and stress resistance among animals cultivated on such diets have been reported.[10] DMS, is responsible for repellent, 'off' tastes and odours that develop in some seafood products because of the action of bacterial DMSP-lyase, which cogenerates acrylate.
Further reading
- Kenji Nakajima (2015). "Amelioration Effect of a Tertiary Sulfonium Compound, Dimethylsulfoniopropionate". In S.-K. Kim (ed.). Handbook of Anticancer Drugs from Marine Origin. Springer. pp. 205–238. doi:10.1007/978-3-319-07145-9_11. ISBN 978-3-319-07144-2.
See also
- CLAW hypothesis, proposing a feedback loop that operates between ocean ecosystems and the Earth's climate
- Coccolithophore, a group of marine unicellular planktonic photosynthetic algae, producer of DMSP
- Dimethyl sulfide, a breakdown product of DMSP along with methanethiol
- Dimethyl selenide, a selenium analogue of DMS produced by bacteria and phytoplankton
- Emiliania huxleyi, a coccolithophorid producing DMSP
References
- "Http 404". Archived from the original on 2013-03-06. Retrieved 2013-02-05.
- "Http 404". Archived from the original on 2013-03-06. Retrieved 2013-02-05.
- DeBose, Jennifer L.; Sean C. Lema; Gabrielle A. Nevitt (2008-03-07). "Dimethylsulfoniopropionate as a foraging cue for reef fishes". Science. 319 (5868): 1356. doi:10.1126/science.1151109. PMID 18323445.Vila-Costa, Maria; Rafel Simo; Hyakubun Harada; Josep M. Gasol; Doris Slezak; Ronald P. Kiene (2006-10-27). "Dimethylsulfoniopropionate uptake by marine phytoplankton". Science. 314 (5799): 652–654. doi:10.1126/science.1131043. PMID 17068265.
- Challenger, Frederick; Margaret Isabel Simpson (1948). "Studies on biological methylation. Part XII. A precursor of the dimethyl sulphide evolved by Polysiphonia fastigiata. Dimethyl-2-carboxyethylsulphonium hydroxide and its salts". Journal of the Chemical Society. 3: 1591–1597. doi:10.1039/JR9480001591. PMID 18101461.
- McNeil, Scott D.; Nuccio, Michael L.; Hanson, Andrew D. (1999). "Betaines and Related Osmoprotectants. Targets for Metabolic Engineering of Stress Resistance". Plant Physiology. 120 (4): 945–949. doi:10.1104/pp.120.4.945. PMC 1539222. PMID 10444077.
- Gage, D. A.; Rhodes, D.; Nolte, K. D.; Hicks, W. A.; Leustek, T.; Cooper, A. J.; Hanson, A. D. (1997-06-26). "A new route for synthesis of dimethylsulphoniopropionate in marine algae". Nature. 387 (6636): 891–894. doi:10.1038/43160. ISSN 0028-0836. PMID 9202120.
- Ledyard, Kathleen M.; Delong, Edward F.; Dacey, John W. H. (1993). "Characterization of a DMSP-degrading bacterial isolate from the Sargasso Sea". Archives of Microbiology. 160 (4): 312–318. doi:10.1007/bf00292083.
- Yoch, D. C. (2002). "Dimethylsulfoniopropionate: Its sources, role in the marine food web, and biological degradation to dimethylsulfide". Appl. Env. Microbiol. 68 (12): 5804–15. doi:10.1128/aem.68.12.5804-5815.2002. PMC 134419. PMID 12450799.
- "DMS: The Climate Gas You've Never Heard of".
- Nakajima, Kenji (1996), "Effects of DMSP and Related Compounds on Behavior, Growth and Stress Resistance of Fish, Amphibians and Crustaceans", in Kiene, R. P.; Visscher, P. T.; Keller, M. D.; Kirst, G. O. (eds.), Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds, Springer, Boston, MA, pp. 165–176, doi:10.1007/978-1-4613-0377-0_15, ISBN 9780306453069