Corpus callosum
The corpus callosum (Latin for "tough body"), also callosal commissure, is a wide, thick nerve tract, consisting of a flat bundle of commissural fibers, beneath the cerebral cortex in the brain. The corpus callosum is only found in placental mammals.[1] It spans part of the longitudinal fissure, connecting the left and right cerebral hemispheres, enabling communication between them. It is the largest white matter structure in the human brain, about ten centimetres in length and consisting of 200–300 million axonal projections.[2][3]
| Corpus callosum | |
|---|---|
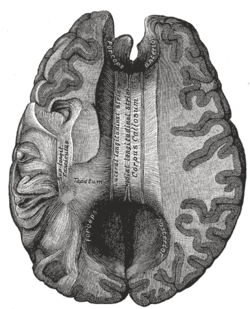 Corpus callosum from above, front part at the top of the image. | |
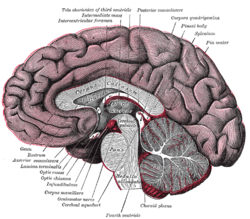 Cross-section of brain, with a person facing forward to the left. The corpus callosum can be seen in the center, in light gray | |
| Details | |
| Pronunciation | /ˈkɔːrpəs kəˈloʊsəm/ |
| Part of | Human brain |
| Parts | Genu, rostrum,trunk, splenium |
| Identifiers | |
| MeSH | D003337 |
| NeuroNames | 191 |
| NeuroLex ID | birnlex_1087 |
| TA | A14.1.09.241 |
| FMA | 86464 |
| Anatomical terms of neuroanatomy | |
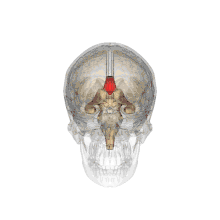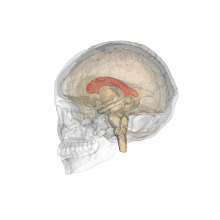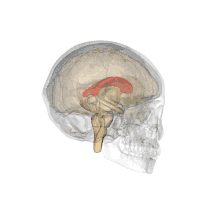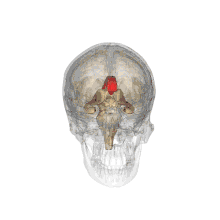
A number of separate nerve tracts, classed as subregions of the corpus callosum, connect different parts of the hemispheres. The main ones are known as the genu, the rostrum, the trunk or body, and the splenium.[4]
Structure
The corpus callosum forms the floor of the longitudinal fissure that separates the two cerebral hemispheres. It also forms part of the roof of the lateral ventricles.[5]
The corpus callosum has four main parts; individual nerve tracts that connect different parts of the hemispheres. These are the rostrum, the genu, the trunk or body, and the splenium.[4] A narrowed part between the trunk and the splenium is known as the isthmus.
The front part of the corpus callosum, towards the frontal lobes is called the genu ("knee"). The genu curves downward and backward in front of the septum pellucidum, diminishing greatly in thickness. The lower much thinner part is the rostrum and is connected below with the lamina terminalis, which stretches from the interventricular foramina to the recess at the base of the optic stalk. The rostrum is named for its resemblance to a bird's beak.
The end part of the corpus callosum, towards the cerebellum, is called the splenium. This is the thickest part, and overlaps the tela choroidea of the third ventricle and the midbrain, and ends in a thick, convex, free border. Splenium translates as bandage in Greek.
The trunk of the corpus callosum lies between the splenium and the genu.
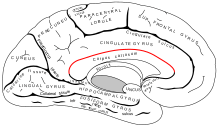
The callosal sulcus separates the corpus callosum from the cingulate gyrus.
Relations
On either side of the corpus callosum, the fibers radiate in the white matter and pass to the various parts of the cerebral cortex; those curving forward from the genu into the frontal lobes constitute the forceps minor (also forceps anterior) and those curving backward from the splenium into the occipital lobes, the forceps major (also forceps posterior).[4] Between these two parts is the main body of the fibers which constitute the tapetum and extend laterally on either side into the temporal lobe, and cover in the central part of the lateral ventricle. The tapetum and anterior commissure share the function of connecting left and right temporal lobes.
The anterior cerebral arteries are in contact with the under surface of the rostrum, they arch over the front of the genu and are carried along the trunk, supplying the front four-fifths of the corpus callosum.[6]
Neuronal fibers
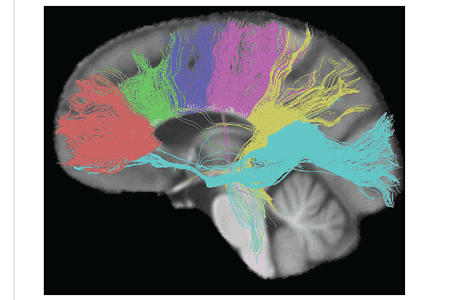
The size, amount of myelination, and density of the fibers in the subregions relate to the functions of the brain regions they connect.[7] Myelination is the process of coating neurons with myelin, which helps the transfer of information between neurons. The process is believed to occur until an individual's thirties with peak growth in the first decade of one’s life.[8] Thinner, lightly myelinated fibers are slower conducting and they connect the association and prefrontal areas. Thicker and fast-conducting fibers connect the visual and motor areas.[9]
The tractogram pictured, shows the nerve tracts from six segments of the corpus callosum, providing linking of the cortical regions between the cerebral hemispheres. Those of the genu are shown in coral, of the premotor – green, of the sensory-motor – purple, of the parietal – pink, of the temporal – yellow, and of the splenium – blue.[10]
Thinner axons in the genu connect the prefrontal cortex between the two halves of the brain; these fibers arise from a fork-like bundle of fibers from the tapetum, the forceps minor. Thicker axons in the trunk of the corpus callosum, interconnect areas of the motor cortex, with proportionately more of the corpus callosum dedicated to supplementary motor regions including Broca's area. The splenium, communicates somatosensory information between the two halves of the parietal lobe and the visual cortex at the occipital lobe, these are the fibers of the forceps major.[11][12]
In a study of five- to eighteen-year-olds there was found to be a positive correlation between age and callosal thickness.[3]
Variation between sexes
The corpus callosum and its relation to sex has been a subject of debate in the scientific and lay communities for over a century. Initial research in the early 20th century claimed the corpus to be different in size between men and women. That research was in turn questioned, and ultimately gave way to more advanced imaging techniques that appeared to refute earlier correlations. However, advanced analytical techniques of computational neuroanatomy developed in the 1990s showed that sex differences were clear but confined to certain parts of the corpus callosum, and that they correlated with cognitive performance in certain tests.[13] One recent study using magnetic resonance imaging (MRI) found that the midsagittal corpus callosum cross-sectional area is, after controlling for brain size, on average, proportionately larger in females.[14]
Using diffusion tensor sequences on MRI machines, the rate at which molecules diffuse in and out of a specific area of tissue, anisotropy can be measured and used as an indirect measurement of anatomical connection strength. These sequences have found consistent sex differences in human corpus callosal shape and microstructure.[15][16][17]
Analysis by shape and size has also been used to study specific three-dimensional mathematical relationships with MRIs, and have found consistent and statistically significant differences across sexes.[18][19] Specific algorithms have found significant differences between the two sexes in over 70% of cases in one review.[20]
Correlates of size with handedness
One study reported that the front portion of the human corpus callosum was 0.75 cm2 or 11% larger in left-handed and ambidextrous people than right-handed people.[21][22] This difference was evident in the anterior and posterior regions of the corpus callosum, but not in the splenium.[21] However, this has been challenged and others have instead suggested that the degree of handedness negatively correlates with the size of the corpus callosum, meaning that individuals who are capable of using both hands with dexterity would have the largest corpus callosum and vice versa for either left or right hand.[23]
Clinical significance
Epilepsy
The symptoms of refractory (difficult to treat) epilepsy can be reduced by cutting through the corpus callosum in an operation known as a corpus callosotomy.[24] This is usually reserved for cases in which complex or grand mal seizures are produced by an epileptogenic focus on one side of the brain, causing an interhemispheric electrical storm. The diagnostic work up for this procedure involves an electroencephalogram, MRI, PET scan, and evaluation by a specialized neurologist, neurosurgeon, psychiatrist, and neuroradiologist before surgery can be considered.[25]
Failure to develop
The formation of the corpus callosum begins with the first midline crossing of pioneer axons around week 12 in the prenatal development of the human,[26] or day 15 in the embryogenesis of the mouse.[27] Agenesis of the corpus callosum (ACC) is a rare congenital disorder that is one of the most common brain malformations observed in human beings,[28] in which the corpus callosum is partially or completely absent. ACC is usually diagnosed within the first two years of life, and may manifest as a severe syndrome in infancy or childhood, as a milder condition in young adults, or as an asymptomatic incidental finding. Initial symptoms of ACC usually include seizures, which may be followed by feeding problems and delays in holding the head erect, sitting, standing, and walking. Other possible symptoms may include impairments in mental and physical development, hand-eye coordination, and visual and auditory memory. Hydrocephaly may also occur. In mild cases, symptoms such as seizures, repetitive speech, or headaches may not appear for years. Some syndromes that are often associated with ACC are Aicardi syndrome, Andermann syndrome, Shapiro syndrome, and acrocallosal syndrome.
ACC is usually not fatal. Treatment usually involves management of symptoms, such as hydrocephaly and seizures, if they occur. Although many children with the disorder lead normal lives and have average intelligence, careful neuropsychological testing reveals subtle differences in higher cortical function compared to individuals of the same age and education without ACC. Children with ACC accompanied by developmental delay and/or seizure disorders should be screened for metabolic disorders.[29]
In addition to agenesis of the corpus callosum, similar conditions are hypogenesis (partial formation), dysgenesis (malformation), and hypoplasia (underdevelopment, including too thin).
Recent studies have also linked possible correlations between corpus callosum malformation and autism spectrum disorders.[30]
Kim Peek, a savant and the inspiration behind the movie Rain Man, was found with agenesis of the corpus callosum, as part of FG syndrome.
Other disease
Anterior corpus callosum lesions may result in akinetic mutism or anomic aphasia. See also:
- Alien hand syndrome
- Alexia without agraphia (seen with damage to splenium of corpus callosum)
- Marchiafava–Bignami disease a degenerative disease characterised by loss of myelin and necrosis of the corpus callosum
- Multiple sclerosis with the Dawson's fingers sign
- Reversible splenial lesion syndrome – a rare encephalopathy of unknown origin with a transient lesion in the splenium, mostly associated with infectious diseases
- Septo-optic dysplasia (deMorsier syndrome)
- Split-brain
- Susac's syndrome characterised by lesions as small holes in the corpus callosum
History
The first study of the corpus with relation to gender was by R. B. Bean, a Philadelphia anatomist, who suggested in 1906 that "exceptional size of the corpus callosum may mean exceptional intellectual activity" and that there were measurable differences between men and women. Perhaps reflecting the political climate of the times, he went on to claim differences in the size of the callosum across different races. His research was ultimately refuted by Franklin Mall, the director of his own laboratory.[31]
Of more mainstream impact was a 1982 Science article by Holloway and Utamsing that suggested sex difference in human brain morphology, which related to differences in cognitive ability.[32] Time published an article in 1992 that suggested that, because the corpus is "often wider in the brains of women than in those of men, it may allow for greater cross-talk between the hemispheres—possibly the basis for women’s intuition."[33]
More recent publications in the psychology literature have raised doubt as to whether the anatomic size of the corpus is actually different. A meta-analysis of 49 studies since 1980 found that, contrary to de Lacoste-Utamsing and Holloway, no sex difference could be found in the size of the corpus callosum, whether or not account was taken of larger male brain size.[31] A study in 2006 using thin slice MRI showed no difference in thickness of the corpus when accounting for the size of the subject.[34]
Other animals
The corpus callosum is found only in placental mammals (the eutherians), while it is absent in monotremes and marsupials,[35] as well as other vertebrates such as birds, reptiles, amphibians and fish.[36] (Other groups do have other brain structures that allow for communication between the two hemispheres, such as the anterior commissure, which serves as the primary mode of interhemispheric communication in marsupials,[37][38] and which carries all the commissural fibers arising from the neocortex (also known as the neopallium), whereas in placental mammals, the anterior commissure carries only some of these fibers.[39]) In primates, the speed of nerve transmission depends on its degree of myelination, or lipid coating. This is reflected by the diameter of the nerve axon. In most primates, axonal diameter increases in proportion to brain size to compensate for the increased distance to travel for neural impulse transmission. This allows the brain to coordinate sensory and motor impulses. However, the scaling of overall brain size and increased myelination have not occurred between chimpanzees and humans. This has resulted in the human corpus callosum's requiring double the time for interhemispheric communication as a macaque's.[11] The fibrous bundle at which the corpus callosum appears, can and does increase to such an extent in humans that it encroaches upon and wedges apart the hippocampal structures.[40]
Additional images
- Corpus callosum
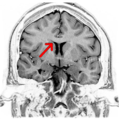 Coronal T2 (grey scale inverted) MRI of the brain at the level of the caudate nuclei emphasizing corpus callosum
Coronal T2 (grey scale inverted) MRI of the brain at the level of the caudate nuclei emphasizing corpus callosum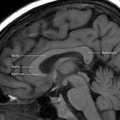 Corpus callosum parts on MRI
Corpus callosum parts on MRI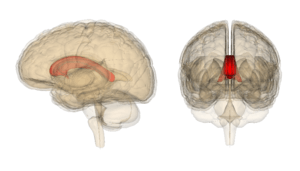 Corpus callosum with Anatomography
Corpus callosum with Anatomography
References
- Velut, S; Destrieux, C; Kakou, M (May 1998). "[Morphologic anatomy of the corpus callosum]". Neuro-Chirurgie. 44 (1 Suppl): 17–30. PMID 9757322.
- "Corpus callosum". Queensland Brain Institute. 10 November 2017.
- Luders, Eileen; Thompson, Paul M.; Toga, Arthur W. (18 August 2010). "The Development of the Corpus Callosum in the Healthy Human Brain". Journal of Neuroscience. 30 (33): 10985–10990. doi:10.1523/JNEUROSCI.5122-09.2010. PMC 3197828. PMID 20720105.
- Gaillard, Frank. "Corpus callosum | Radiology Reference Article | Radiopaedia.org". radiopaedia.org.
- Carpenter, Malcolm (1985). Core text of neuroanatomy (3rd ed.). Baltimore: Williams & Wilkins. pp. 26–32. ISBN 978-0683014556.
- Ropper, A.; Samuels, M.; Klein, J. (2014). Adams and Victor's Principles of Neurology (10th ed.). McGraw-Hill. p. 798. ISBN 978-0071794794.
- Doron, KW; Gazzaniga, MS (September 2008). "Neuroimaging techniques offer new perspectives on callosal transfer and interhemispheric communication". Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 44 (8): 1023–9. doi:10.1016/j.cortex.2008.03.007. PMID 18672233.
- Schlaug, Gotfried; Jäncke, Lutz; Huang, Yanxiong; Staiger, Jochen F; Steinmetz, Helmuth (April 10, 2010). "Increased Corpus Callosum Size in Musicians". Neuropsychologia. 25 (4): 557–577. doi:10.1177/0743558410366594.
- Aboitiz, F (1992). "Brain connections: interhemispheric fiber systems and anatomical brain asymmetries in humans". Biological Research. 25 (2): 51–61. PMID 1365702.
- "NIAAA Publications". pubs.niaaa.nih.gov.
- Caminiti, Roberto; Ghaziri, Hassan; Galuske, Ralf; Hof, Patrick R.; Innocenti, Giorgio M. (2009). "Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates". Proceedings of the National Academy of Sciences. 106 (46): 19551–6. Bibcode:2009PNAS..10619551C. doi:10.1073/pnas.0907655106. JSTOR 25593230. PMC 2770441. PMID 19875694.
- Hofer, Sabine; Frahm, Jens (2006). "Topography of the human corpus callosum revisited—Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging". NeuroImage. 32 (3): 989–94. doi:10.1016/j.neuroimage.2006.05.044. PMID 16854598.
- Davatzikos, C; Resnick, S. M. (1998). "Sex differences in anatomic measures of interhemispheric connectivity: Correlations with cognition in women but not men". Cerebral Cortex. 8 (7): 635–40. doi:10.1093/cercor/8.7.635. PMID 9823484.
- Ardekani, B. A.; Figarsky, K.; Sidtis, J. J. (2012). "Sexual Dimorphism in the Human Corpus Callosum: An MRI Study Using the OASIS Brain Database". Cerebral Cortex. 23 (10): 2514–20. doi:10.1093/cercor/bhs253. PMC 3767965. PMID 22891036.
- Dubb, Abraham; Gur, Ruben; Avants, Brian; Gee, James (2003). "Characterization of sexual dimorphism in the human corpus callosum". NeuroImage. 20 (1): 512–9. doi:10.1016/S1053-8119(03)00313-6. PMID 14527611.
- Westerhausen, René; Kreuder, Frank; Sequeira, Sarah Dos Santos; Walter, Christof; Woerner, Wolfgang; Wittling, Ralf Arne; Schweiger, Elisabeth; Wittling, Werner (2004). "Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: A combined high-resolution and diffusion-tensor MRI study". Cognitive Brain Research. 21 (3): 418–26. doi:10.1016/j.cogbrainres.2004.07.002. PMID 15511657.
- Shin, Yong-Wook; Jin Kim, Dae; Hyon Ha, Tae; Park, Hae-Jeong; Moon, Won-Jin; Chul Chung, Eun; Min Lee, Jong; Young Kim, In; Kim, Sun I.; et al. (2005). "Sex differences in the human corpus callosum: Diffusion tensor imaging study". NeuroReport. 16 (8): 795–8. doi:10.1097/00001756-200505310-00003. PMID 15891572.
- Kontos, Despina; Megalooikonomou, Vasileios; Gee, James C. (2009). "Morphometric analysis of brain images with reduced number of statistical tests: A study on the gender-related differentiation of the corpus callosum". Artificial Intelligence in Medicine. 47 (1): 75–86. doi:10.1016/j.artmed.2009.05.007. PMC 2732126. PMID 19559582.
- Spasojevic, Goran; Stojanovic, Zlatan; Suscevic, Dusan; Malobabic, Slobodan (2006). "Sexual dimorphism of the human corpus callosum: Digital morphometric study". Vojnosanitetski Pregled. 63 (11): 933–8. doi:10.2298/VSP0611933S. PMID 17144427.
- Yokota, Y.; Kawamura, Y.; Kameya, Y. (2005). Callosal Shapes at the Midsagittal Plane: MRI Differences of Normal Males, Normal Females, and GID. 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference. 3. pp. 3055–8. doi:10.1109/IEMBS.2005.1617119. ISBN 978-0-7803-8741-6. PMID 17282888.
- Witelson, S. (1985). "The brain connection: The corpus callosum is larger in left-handers". Science. 229 (4714): 665–8. Bibcode:1985Sci...229..665W. doi:10.1126/science.4023705. PMID 4023705.
- Driesen, Naomi R.; Raz, Naftali (1995). "The influence of sex, age, and handedness on corpus callosum morphology: A meta-analysis". Psychobiology. 23 (3): 240–7.
- Luders, Eileen; Cherbuin, Nicolas; Thompson, Paul M.; Gutman, Boris; Anstey, Kaarin J.; Sachdev, Perminder; Toga, Arthur W. (2010-08-01). "When more is less: Associations between corpus callosum size and handedness lateralization". NeuroImage. 52 (1): 43–49. doi:10.1016/j.neuroimage.2010.04.016. ISSN 1053-8119.
- Clarke, Dave F.; Wheless, James W.; Chacon, Monica M.; Breier, Joshua; Koenig, Mary-Kay; McManis, Mark; Castillo, Edward; Baumgartner, James E. (2007). "Corpus callosotomy: A palliative therapeutic technique may help identify resectable epileptogenic foci". Seizure. 16 (6): 545–53. doi:10.1016/j.seizure.2007.04.004. PMID 17521926.
- "WebMd Corpus Callotomy". Web MD. July 18, 2010. Archived from the original on July 2, 2010. Retrieved July 18, 2010.
- Rakic, P; Yakovlev, PI (January 1968). "Development of the corpus callosum and cavum septi in man". The Journal of Comparative Neurology. 132 (1): 45–72. doi:10.1002/cne.901320103. PMID 5293999.
- Rash, BG; Richards, LJ (28 May 2001). "A role for cingulate pioneering axons in the development of the corpus callosum". The Journal of Comparative Neurology. 434 (2): 147–57. doi:10.1002/cne.1170. PMID 11331522.
- Dobyns, W. B. (1996). "Absence makes the search grow longer". American Journal of Human Genetics. 58 (1): 7–16. PMC 1914936. PMID 8554070.
- "NINDS Agenesis of the Corpus Callosum Information Page: NINDS". RightDiagnosis.com. Archived from the original on 2012-03-24. Retrieved Aug 30, 2011.
- "Autism May Involve A Lack Of Connections And Coordination In Separate Areas Of The Brain, Researchers Find". Medical News Today. Archived from the original on 2011-10-15.
- Bishop, Katherine M.; Wahlsten, Douglas (1997). "Sex Differences in the Human Corpus Callosum: Myth or Reality?" (PDF). Neuroscience & Biobehavioral Reviews. 21 (5): 581–601. doi:10.1016/S0149-7634(96)00049-8. PMID 9353793.
- Delacoste-Utamsing, C; Holloway, R. (1982). "Sexual dimorphism in the human corpus callosum". Science. 216 (4553): 1431–2. Bibcode:1982Sci...216.1431D. doi:10.1126/science.7089533. PMID 7089533.
- C Gorman (20 January 1992). "Sizing up the sexes". Time: 36–43. As cited by Bishop and Wahlsten.
- Luders, Eileen; Narr, Katherine L.; Zaidel, Eran; Thompson, Paul M.; Toga, Arthur W. (2006). "Gender effects on callosal thickness in scaled and unscaled space". NeuroReport. 17 (11): 1103–6. doi:10.1097/01.wnr.0000227987.77304.cc. PMID 16837835. S2CID 14466914.
- Keeler, Clyde E. (1933). "Absence of the Corpus callosum as a Mendelizing Character in the House Mouse". Proceedings of the National Academy of Sciences of the United States of America. 19 (6): 609–11. Bibcode:1933PNAS...19..609K. doi:10.1073/pnas.19.6.609. JSTOR 86284. PMC 1086100. PMID 16587795.
- Sarnat, Harvey B., and Paolo Curatolo (2007). Malformations of the Nervous System: Handbook of Clinical Neurology, p. 68
- Ashwell, Ken (2010). The Neurobiology of Australian Marsupials: Brain Evolution in the Other Mammalian Radiation, p. 50
- Armati, Patricia J., Chris R. Dickman, and Ian D. Hume (2006). Marsupials, p. 175
- Butler, Ann B., and William Hodos (2005). Comparative Vertebrate Neuroanatomy: Evolution and Adaptation, p. 361
- Morris, H., & Schaeffer, J. P. (1953). The Nervous system-The Brain or Encephalon. Human anatomy; a complete systematic treatise. (11th ed., pp. 920–921, 964–965). New York: Blakiston.
External links
| Wikimedia Commons has media related to Corpus callosum. |