9-Borabicyclo(3.3.1)nonane
9-Borabicyclo[3.3.1]nonane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates.[1] 9-BBN is also known by its nickname 'banana borane'.[2] This is because rather than drawing out the full structure, chemists often simply draw a banana shape with the bridging boron.[3]
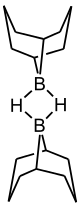 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
9-Borabicyclo[3.3.1]nonane | |
| Other names
Borabicyclononane Banana borane | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | 9-BBN |
| ChemSpider | |
| ECHA InfoCard | 100.005.456 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H30B2 | |
| Molar mass | 244.04 g·mol−1 |
| Density | 0.894 g/cm3 |
| Melting point | 153 to 155 °C (307 to 311 °F; 426 to 428 K) |
| Reacts | |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H250, H260, H314 |
| P210, P222, P223, P231+232, P260, P264, P280, P301+330+331, P302+334, P303+361+353, P304+340, P305+351+338, P310, P321, P335+334, P363, P370+378, P402+404, P405, P422, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
9-BBN is prepared by the reaction of 1,5-cyclooctadiene and borane usually in ethereal solvents, for example:[4][5]
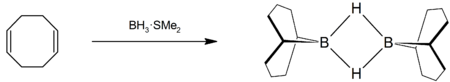
The compound is commercially available as a solution in tetrahydrofuran and as a solid. 9-BBN is especially useful in Suzuki reactions.[6][7][8]
Its highly regioselective addition on alkenes allows the preparation of terminal alcohols by subsequent oxidative cleavage with H2O2 in aqueous KOH. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane.
References
- Brown, H. C. (1975). Organic Syntheses via Boranes. New York: John Wiley & Sons. ISBN 0-471-11280-1.
- Stix, Gary. "The Straight Dope: A Q&A with the Prof behind the Good Science in Breaking Bad". Scientific American Blog Network. Nature America, Inc. Retrieved 18 June 2017.
- "Molecules with Silly or Unusual Names - page 3". Chm.bris.ac.uk. 2014-05-07. Retrieved 2016-06-01.
- Soderquist, John A.; Brown, Herbert C. (1981). "Simple, remarkably efficient route to high purity, crystalline 9-borabicyclo[3.3.1]nonane (9-BBN) dimer". J. Org. Chem. 46 (22): 4599–4600. doi:10.1021/jo00335a067.
- Soderquist, John A.; Alvin, Negron (1998). "9-Borabicyclo[3.3.1]nonane Dimer". Organic Syntheses.; Collective Volume, 9, p. 95
- Ishiyama, Tatsuo; Miyaura, Norio; Suzuki, Akira. "Palladium(0)-catalyzed reaction of 9-alkyl-9-borabicyclo[3.3.1]nonane with 1-bromo-1-phenylthioethene: 4-(3-cyclohexenyl)-2-phenylthio-1-butene". Organic Syntheses.; Collective Volume, 9, p. 107
- Balog, A.; Meng, D.; Kamenecka, T.; Bertinato, P.; Su, D.-S.; Sorensen, E. J.; Danishefsky, S. J. (1996). "Total Synthesis of (−)-Epothilone A". Angew. Chem. Int. Ed. Engl. 35: 2801. doi:10.1002/anie.199628011.
- Liu, J.; Lotesta, S. D.; Sorensen, E. J. (2011). "A concise synthesis of the molecular framework of pleuromutilin". Chem. Commun. 47: 1500. doi:10.1039/C0CC04077K. PMC 3156455. PMID 21079876.