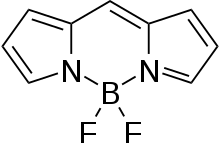
BODIPY
BODIPY is the technical common name of a chemical compound with formula C
9H
7BN
2F
2, whose molecule consists of a boron difluoride group BF
2 joined to a dipyrromethene group C
9H
7N
2; specifically, the compound 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene in the IUPAC nomenclature.[1] The common name is an abbreviation for "boron-dipyrromethene". It is a red crystalline solid, stable at ambient temperature, soluble in methanol.[1]
 | |
| Names | |
|---|---|
| IUPAC name
4,4-difluoro-4-bora-3a,4a-diaza-s-indacene | |
| Other names
dipyrrometheneboron difluoride | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C9H7BF2N2 | |
| Molar mass | 191.98 g·mol−1 |
| Appearance | red crystalline solid [1] |
| Melting point | 450 °C [1] |
| Solubility | methanol, dichloromethane[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |

The compound itself was isolated only in 2009,[2][1][3] but many derivatives—formally obtained by replacing one or more hydrogen atoms by other functional groups—have been known since 1968, and comprise the important class of BODIPY dyes.[4] These organoboron compounds have attracted much interest as fluorescent dyes and markers in biological research.[1]
Structure
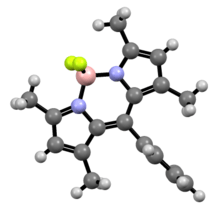
In its crystalline solid form, the core BODIPY is almost, but not entirely, planar and symmetrical; except for the two fluorine atoms, that lie on the perpendicular bisecting plane.[1]. Its bonding can be explained by assuming a formal negative charge on the boron atom, and a formal positive charge on one of the nitrogen atoms.
Synthesis
BODIPY and its derivatives can be obtained by reacting the corresponding 2,2'-dipyrromethene derivatives with boron trifluoride-diethyl ether complex (BF
3·(C
2H
5)
2O) in the presence of triethylamine or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU).[1] The difficulty of the synthesis was due to instability of the usual dipyrromethene precursor, rather than of BODIPY itself.[1][5]
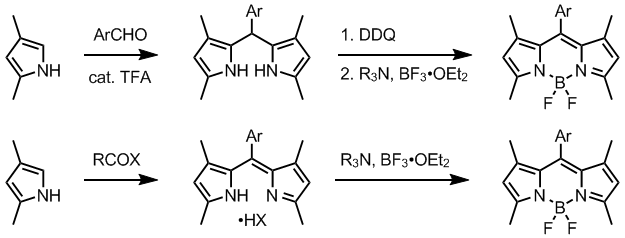
The dipyrromethene precursors are accessed from a suitable pyrrole derivatives by several methods. Normally, one alpha-position in employed pyrroles is substituted and the other is free. Condensation of such pyrrole, often available from Knorr pyrrole synthesis, with an aromatic aldehyde in the presence of TFA gives dipyrromethane, which is oxidized to dipyrromethene using a quinone oxidant such as DDQ[1] or p-chloranil.[6]
Alternatively, dipyrromethenes are prepared by treating a pyrrole with an activated carboxylic acid derivative, usually an acyl chloride. Unsymmetrical dipyrromethenes can be obtained by condensing pyrroles with 2-acylpyrroles. Intermediate dipyrromethanes may be isolated and purified, but isolation of dipyrromethenes is usually compromised by their instability.
Derivatives

The BODIPY core has a rich derivative chemistry due to the high tolerance for substitutions in the pyrrole and aldehyde (or acyl chloride) starting materials.[5]
Hydrogen atoms at the 2 and 6 positions of the cyclic core can be displaced by halogen atoms using succinimide reagents such as NCS, NBS and NIS - which allows for further post-functionalisation through palladium coupling reactions with boronate esters, tin reagents etc.[5]
The two fluorine atoms on the boron atom can be replaced, during or after synthesis, by other strong nucleophilic reagents, such as lithiated alkyne or aryl species,[5] chlorine,[6] methoxy,[6] or a divalent "strap".[9] The reaction is catalysed by BBr3 or SnCl4[10].
Fluorescence
BODIPY and many of its derivatives have received attention recently for being fluorescent dyes with unique properties. They strongly absorb UV-radiation and re-emit it in very narrow frequency spreads, with high quantum yields, mostly at wavelengths below 600 nm. They are relatively insensitive to the polarity and pH of their environment and are reasonably stable to physiological conditions. Small modifications to their structures enable tuning of their fluorescence characteristics.[7] BODIPY dyes are relatively chemically inert. Fluorescence is quenched in a solution, which limits application. This problem has been handled by synthesizing asymmetric boron complexes and replacing the fluorine groups with phenyl groups.
The unsubstituted BODIPY has a broad absorption band, from about 420 to 520 nm (peaking at 503 nm) and a broad emission band from about 480 to 580 nm (peaking at 512 nm), with a fluorescence lifetime of 7.2 ns. Its fluorescence quantum yield is near 1, greater than that of substituted BODIPY dyes and comparable to those of rhodamine and fluoresceine, but fluorescence is lost above 50 °C.[2]
BODIPY dyes are notable for their uniquely small Stokes shift, high, environment-independent fluorescence quantum yields, often approaching 100% even in water, sharp excitation and emission peaks contributing to overall brightness, and high solubility in many organic solvents. The combination of these qualities makes BODIPY fluorophores promising for imaging applications. The position of the absorption and emission bands remain almost unchanged in solvents of different polarity as the dipole moment and transition dipole are mutually orthogonal.
Potential applications
BODIPY conjugates are widely studied as potential sensors and for labelling by exploiting its highly tunable optoelectronic properties.[11] [12] [13] [14] [15][16] [17] [18]
References
- K. Tram; H. Yan; H. A. Jenkins; S. Vassiliev; D. Bruce (2009). "The synthesis and crystal structure of unsubstituted 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY)". Dyes and Pigments. 82 (3): 392–395. doi:10.1016/j.dyepig.2009.03.001.
- A. Schmitt; B. Hinkeldey; M. Wild; G. Jung (2009). "Synthesis of the Core Compound of the BODIPY Dye Class: 4,4′-Difluoro-4-bora-(3a,4a)-diaza-s-indacene". J. Fluoresc. 19 (4): 755–759. doi:10.1007/s10895-008-0446-7. PMID 19067126.
- I. J. Arroyo; R. Hu; G. Merino; B. Z. Tang; E. Peña-Cabrera (2009). "The Smallest and One of the Brightest. Efficient Preparation and Optical Description of the Parent Borondipyrromethene System". J. Org. Chem. 74 (15): 5719–22. doi:10.1021/jo901014w. PMID 19572588.
- Alfred Treibs und Franz-Heinrich Kreuzer. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Annalen der Chemie 1968, 718 (1): 208-223.
- Burgess, Kevin (October 2007). "BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties". Chemical Reviews. 107 (11): 4891–4932. doi:10.1021/cr078381n. PMID 17924696.
- Brandon R. Groves, Sarah M. Crawford,a Travis Lundrigan, Chérif F. Matta, Shahin Sowlati-Hashjin, and Alison Thompson (2013): "Synthesis and characterisation of the unsubstituted dipyrrin and 4,4-dichloro-4-bora-3a,4a-diaza-s-indacene: improved synthesis and functionalisation of the simplest BODIPY framework." Chemical Communications, volume 49, pages 816-818. doi:10.1039/c2cc37480c
- Aurore Loudet and Kevin Burgess (2007): "BODIPY dyes and their derivatives: Syntheses and spectroscopic properties". Chemical Reviews, volume 107, issue 11, pages 4891–4932. doi:10.1021/cr078381n
- Zhong-Hua Pan; Geng-Geng Luo; Jing-Wei Zhou; Jiu-Xu Xia; Kai Fang; Rui-Bo Wu (2014). "A simple BODIPY-aniline-based fluorescent chemosensor as multiple logic operations for the detection of pH and CO2 gas". Dalton Trans. 43 (22): 8499. doi:10.1039/C4DT00395K.
- Stachelek, Patrycja; Alsimaree, Abdulrahman A.; Alnoman, Rua B.; Harriman, Anthony; Knight, Julian G. (2017-03-16). "Thermally-Activated, Delayed Fluorescence in O,B,O- and N,B,O-Strapped Boron Dipyrromethene Derivatives". The Journal of Physical Chemistry A. 121 (10): 2096–2107. doi:10.1021/acs.jpca.6b11131. ISSN 1089-5639.
- Sirbu, Dumitru; Benniston, Andrew C.; Harriman, Anthony (2017-04-07). "One-Pot Synthesis of a Mono-O,B,N-strapped BODIPY Derivative Displaying Bright Fluorescence in the Solid State". Organic Letters. 19 (7): 1626–1629. doi:10.1021/acs.orglett.7b00435. ISSN 1523-7060.
- Sirbu, Dumitru; Luli, Saimir; Leslie, Jack; Oakley, Fiona; Benniston, Andrew C. (2019-05-17). "Enhanced in vivo Optical Imaging of the Inflammatory Response to Acute Liver Injury in C57BL/6 Mice Using a Highly Bright Near-Infrared BODIPY Dye". ChemMedChem. 14 (10): 995–999. doi:10.1002/cmdc.201900181. ISSN 1860-7179.
- Kowada, Toshiyuki; Maeda, Hiroki; Kikuchi, Kazuya (2015). "BODIPY-based probes for the fluorescence imaging of biomolecules in living cells". Chemical Society Reviews. 44 (14): 4953–4972. doi:10.1039/C5CS00030K. PMID 25801415.
- Ni, Yong; Wu, Jishan (2014). "Far-red and near infrared BODIPY dyes: Synthesis and applications for fluorescent pH probes and bio-imaging". Organic & Biomolecular Chemistry. 12 (23): 3774. doi:10.1039/c3ob42554a. PMID 24781214.
- Abrahamse, Heidi; Hamblin, Michael R. (2016). "New photosensitizers for photodynamic therapy". Biochemical Journal. 473 (4): 347–364. doi:10.1042/BJ20150942. PMC 4811612. PMID 26862179.
- Kowada, Toshiyuki; Maeda, Hiroki; Kikuchi, Kazuya (2015). "BODIPY-based probes for the fluorescence imaging of biomolecules in living cells". Chemical Society Reviews. 44 (14): 4953–4972. doi:10.1039/C5CS00030K. PMID 25801415.
- Lu, Hua; Mack, John; Yang, Yongchao; Shen, Zhen (2014). "Structural modification strategies for the rational design of red/NIR region BODIPYs". Chem. Soc. Rev. 43 (13): 4778–4823. doi:10.1039/C4CS00030G. PMID 24733589.
- Roncali, Jean (2009). "Molecular Bulk Heterojunctions: An Emerging Approach to Organic Solar Cells". Accounts of Chemical Research. 42 (11): 1719–1730. doi:10.1021/ar900041b. PMID 19580313.
- Kim, Ha Na; Ren, Wen Xiu; Kim, Jong Seung; Yoon, Juyoung (2012). "Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions". Chem. Soc. Rev. 41 (8): 3210–3244. doi:10.1039/C1CS15245A. PMID 22184584.