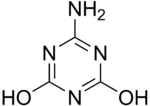
Ammelide
Ammelide (6-amino-2,4-dihydroxy-1,3,5-triazine) is a triazine and the hydrolysis product of ammeline.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
6-Amino-2,4-dihydroxy-1,3,5-triazine | |
| Other names
Ammelid, 2-Amino-1,3,5-triazine-4,6-dione, 2-Amino-4,6-dihydroxy-s-triazine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.416 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4N4O2 | |
| Molar mass | 128.09 g/mol |
| Appearance | white powder |
| insoluble | |
| Solubility | soluble in concentrated mineral acids, alkalis and ammonia |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Ammelide can be obtained by heating dicyandiamide with aqueous ammonia at 160−170 °C. It can also be synthesized by heating melam with concentrated sulfuric acid for a short time at 190 °C.
Chemical property
Ammelide forms salts with both acids (hydrochloric acid, nitric acid, sulfuric acid) and bases (sodium hydroxide, ammonium, calcium hydroxide).
Ammelide decomposes at 170 °C with water to form carbon dioxide and ammonia. It can be converted into cyanuric acid by oxidizing agents (e.g. potassium permanganate) or by boiling with acids or alkalis.
gollark: Nope. It uses the server to server protocol.
gollark: The Pi has a publicly routable and accessible IPv6 address.
gollark: Strictly speaking, yes.
gollark: But all is to be APIONET?
gollark: I mean, we had [REDACTED] incidents with it, but that was just due to me unplugging it.
References
- B. Bann and S.A. Miller, "Melamines and derivatives of melamine", Chemical Reviews, vol.58, p131-172 (1958).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.