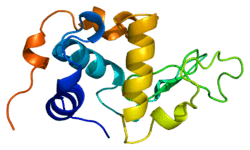Alpha-lactalbumin
Lactalbumin, alpha-, also known as LALBA, is a protein that in humans is encoded by the LALBA gene.[5][6][7]
Function
α-Lactalbumin is a protein that regulates the production of lactose in the milk of almost all mammalian species.[8] In primates, alpha-lactalbumin expression is upregulated in response to the hormone prolactin and increases the production of lactose.[9]
α-Lactalbumin forms the regulatory subunit of the lactose synthase (LS) heterodimer and β-1,4-galactosyltransferase (beta4Gal-T1) forms the catalytic component. Together, these proteins enable LS to produce lactose by transferring galactose moieties to glucose. As a multimer, alpha-lactalbumin strongly binds calcium and zinc ions and may possess bactericidal or antitumor activity. A folding variant of human alpha-lactalbumin that may form in acidic environments such as the stomach, called HAMLET, probably induces apoptosis in tumor and immature cells.[5] The corresponding folding dynamics of alpha-lactalbumin is thus highly unusual.[10]
When formed into a complex with Gal-T1, a galactosyltransferase, α-lactalbumin, enhances the enzyme's affinity for glucose by about 1000 times, and inhibits the ability to polymerise multiple galactose units. This gives rise to a pathway for forming lactose by converting Gal-TI to Lactose synthase.
Physical properties
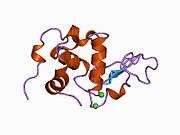
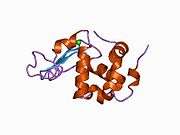
The structure of alpha-lactalbumin is well known and is composed of 123 amino acids and 4 disulfide bridges. The molecular weight is 14178 Da, and the isoelectric point is between 4.2 and 4.5. One of the main structural differences with beta-lactoglobulin is that it does not have any free thiol group that can serve as the starting-point for a covalent aggregation reaction. As a result, pure α-lactalbumin will not form gels upon denaturation and acidification.
Evolution
The sequence comparison of α-lactalbumin shows a strong similarity to that of lysozymes, specifically the Ca2+-binding c-lysozyme.[11] So the expected evolutionary history is that gene duplication of the c-lysozyme was followed by mutation.[8] This gene predates the last common ancestor of mammals and birds, which probably puts its origin at about 300 Ma.[12]
References
- GRCh38: Ensembl release 89: ENSG00000167531 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000022991 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: LALBA lactalbumin, alpha-".
- Hall L, Davies MS, Craig RK (January 1981). "The construction, identification and characterisation of plasmids containing human alpha-lactalbumin cDNA sequences". Nucleic Acids Res. 9 (1): 65–84. doi:10.1093/nar/9.1.65. PMC 326669. PMID 6163135.
- Hall L, Emery DC, Davies MS, Parker D, Craig RK (March 1987). "Organization and sequence of the human alpha-lactalbumin gene". Biochem. J. 242 (3): 735–42. PMC 1147772. PMID 2954544.
- Qasba PK, Kumar S (1997). "Molecular divergence of lysozymes and alpha-lactalbumin". Crit. Rev. Biochem. Mol. Biol. 32 (4): 255–306. doi:10.3109/10409239709082574. PMID 9307874.
- Kleinberg JL, Todd J, Babitsky G (1983). "Inhibition by estradiol of the lactogenic effect of prolactin in primate mammary tissue: reversal by antiestrogens LY 156758 and tamoxifen". PNAS. 80 (13): 4144–4148. doi:10.1073/pnas.80.13.4144. PMC 394217. PMID 6575400.
- Bu, Z.; Cook, J.; Callaway, D. J. E. (2001). "Dynamic regimes and correlated structural dynamics in native and denatured alpha-lactalbumin". J. Mol. Biol. 312 (4): 865–873. doi:10.1006/jmbi.2001.5006. PMID 11575938.
- Acharya KR, Stuart DI, Walker NP, Lewis M, Phillips DC (1989). "Refined structure of baboon alpha-lactalbumin at 1.7 A resolution. Comparison with C-type lysozyme". J. Mol. Biol. 208 (1): 99–127. doi:10.1016/0022-2836(89)90091-0. PMID 2769757.
- Prager EM, Wilson AC (1988). "Ancient origin of lactalbumin from lysozyme: analysis of DNA and amino acid sequences". J. Mol. Evol. 27 (4): 326–35. doi:10.1007/BF02101195. PMID 3146643.
Further reading
- Heine WE, Klein PD, Reeds PJ (1991). "The importance of alpha-lactalbumin in infant nutrition". J. Nutr. 121 (3): 277–83. PMID 2002399.
- Permyakov EA, Berliner LJ (2000). "alpha-Lactalbumin: structure and function". FEBS Lett. 473 (3): 269–74. doi:10.1016/S0014-5793(00)01546-5. PMID 10818224.
- Hall L, Emery DC, Davies MS, et al. (1987). "Organization and sequence of the human alpha-lactalbumin gene". Biochem. J. 242 (3): 735–42. PMC 1147772. PMID 2954544.
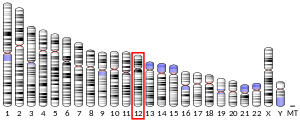
- Davies MS, West LF, Davis MB, et al. (1987). "The gene for human alpha-lactalbumin is assigned to chromosome 12q13". Ann. Hum. Genet. 51 (Pt 3): 183–8. doi:10.1111/j.1469-1809.1987.tb00869.x. PMID 3479943.
- Findlay JB, Brew K (1972). "The complete amino-acid sequence of human -lactalbumin". Eur. J. Biochem. 27 (1): 65–86. doi:10.1111/j.1432-1033.1972.tb01812.x. PMID 5049057.
- Hall L, Craig RK, Edbrooke MR, Campbell PN (1982). "Comparison of the nucleotide sequence of cloned human and guinea-pig pre-alpha-lactalbumin cDNA with that of chick pre-lysozyme cDNA suggests evolution from a common ancestral gene". Nucleic Acids Res. 10 (11): 3503–3515. doi:10.1093/nar/10.11.3503. PMC 320727. PMID 6285305.
- Håkansson A, Zhivotovsky B, Orrenius S, et al. (1995). "Apoptosis induced by a human milk protein". Proc. Natl. Acad. Sci. U.S.A. 92 (17): 8064–8068. doi:10.1073/pnas.92.17.8064. PMC 41287. PMID 7644538.
- Stacey A, Schnieke A, Kerr M, et al. (1995). "Lactation is disrupted by alpha-lactalbumin deficiency and can be restored by human alpha-lactalbumin gene replacement in mice". Proc. Natl. Acad. Sci. U.S.A. 92 (7): 2835–2839. doi:10.1073/pnas.92.7.2835. PMC 42313. PMID 7708733.
- Fujiwara Y, Miwa M, Takahashi R, et al. (1997). "Position-independent and high-level expression of human alpha-lactalbumin in the milk of transgenic rats carrying a 210-kb YAC DNA". Mol. Reprod. Dev. 47 (2): 157–63. doi:10.1002/(SICI)1098-2795(199706)47:2<157::AID-MRD5>3.0.CO;2-L. PMID 9136116.
- Lindner RA, Kapur A, Carver JA (1997). "The interaction of the molecular chaperone, alpha-crystallin, with molten globule states of bovine alpha-lactalbumin". J. Biol. Chem. 272 (44): 27722–9. doi:10.1074/jbc.272.44.27722. PMID 9346914.
- Giuffrida MG, Cavaletto M, Giunta C, et al. (1998). "The unusual amino acid triplet Asn-Ile-Cys is a glycosylation consensus site in human alpha-lactalbumin". J. Protein Chem. 16 (8): 747–53. doi:10.1023/A:1026359715821. PMID 9365923.
- Chandra N, Brew K, Acharya KR (1998). "Structural evidence for the presence of a secondary calcium binding site in human alpha-lactalbumin". Biochemistry. 37 (14): 4767–4772. doi:10.1021/bi973000t. PMID 9537992.
- Håkansson A, Andréasson J, Zhivotovsky B, et al. (1999). "Multimeric alpha-lactalbumin from human milk induces apoptosis through a direct effect on cell nuclei". Exp. Cell Res. 246 (2): 451–60. doi:10.1006/excr.1998.4265. PMID 9925761.
- Svensson M, Sabharwal H, Håkansson A, et al. (1999). "Molecular characterization of alpha-lactalbumin folding variants that induce apoptosis in tumor cells". J. Biol. Chem. 274 (10): 6388–6396. doi:10.1074/jbc.274.10.6388. PMID 10037730.
- Harata K, Abe Y, Muraki M (1999). "Crystallographic evaluation of internal motion of human alpha-lactalbumin refined by full-matrix least-squares method". J. Mol. Biol. 287 (2): 347–58. doi:10.1006/jmbi.1999.2598. PMID 10080897.
- Last AM, Schulman BA, Robinson CV, Redfield C (2001). "Probing subtle differences in the hydrogen exchange behavior of variants of the human alpha-lactalbumin molten globule using mass spectrometry". J. Mol. Biol. 311 (4): 909–19. doi:10.1006/jmbi.2001.4911. PMID 11518539.
- Bai P, Peng Z (2001). "Cooperative folding of the isolated alpha-helical domain of hen egg-white lysozyme". J. Mol. Biol. 314 (2): 321–9. doi:10.1006/jmbi.2001.5122. PMID 11718563.
- Andrews P (1970). "Purification of lactose synthetase a protein from human milk and demonstration of its interaction with alpha-lactalbumin". FEBS Letters. 9 (5): 297–300. doi:10.1016/0014-5793(70)80382-9. PMID 11947697.
External links
- alpha-Lactalbumin at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human LALBA genome location and LALBA gene details page in the UCSC Genome Browser.