Acesulfame potassium
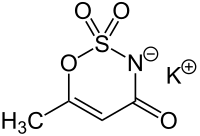
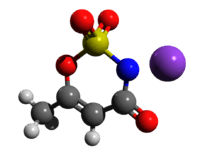
Acesulfame potassium (/ˌeɪsiːˈsʌlfeɪm/ AY-see-SUL-faym[1]), also known as acesulfame K (K is the symbol for potassium) or Ace K, is a calorie-free sugar substitute (artificial sweetener) often marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number (additive code) E950.[2] It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG (now Nutrinova).[3] In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C
4H
4KNO
4S and a molecular weight of 201.24 g/mol.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Potassium 6-methyl-2,2-dioxo-2H-1,2λ6,3-oxathiazin-4-olate | |
| Other names
Acesulfame K; Ace K | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.054.269 |
| EC Number |
|
| E number | E950 (glazing agents, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4KNO4S | |
| Molar mass | 201.242 |
| Appearance | white crystalline powder |
| Density | 1.81 g/cm3 |
| Melting point | 225 °C (437 °F; 498 K) |
| 270 g/L at 20 °C | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
Acesulfame K is 200 times sweeter than sucrose (common sugar), as sweet as aspartame, about two-thirds as sweet as saccharin, and one-third as sweet as sucralose. Like saccharin, it has a slightly bitter aftertaste, especially at high concentrations. Kraft Foods patented the use of sodium ferulate to mask acesulfame's aftertaste.[5] Acesulfame K is often blended with other sweeteners (usually sucralose or aspartame). These blends are reputed to give a more sucrose-like taste whereby each sweetener masks the other's aftertaste, or exhibits a synergistic effect by which the blend is sweeter than its components.[6] Acesulfame potassium has a smaller particle size than sucrose, allowing for its mixtures with other sweeteners to be more uniform.[7]
Unlike aspartame, acesulfame K is stable under heat, even under moderately acidic or basic conditions, allowing it to be used as a food additive in baking, or in products that require a long shelf life. Although acesulfame potassium has a stable shelf life, it can eventually degrade to acetoacetamide, which is toxic in high doses.[8] In carbonated drinks, it is almost always used in conjunction with another sweetener, such as aspartame or sucralose. It is also used as a sweetener in protein shakes and pharmaceutical products,[9] especially chewable and liquid medications, where it can make the active ingredients more palatable. The acceptable daily intake of acesulfame potassium is listed as 15 mg/kg/day.[10]
Acesulfame potassium is widely used in the human diet and excreted by the kidneys. It thus has been used by researchers as a marker to estimate to what degree swimming pools are contaminated by urine.[11]
Other names for acesulfame K are potassium acesulfamate, potassium salt of 6-methyl-1,2,3-oxothiazin-4(3H)-one-2,3-dioxide, and potassium 6-methyl-1,2,3-oxathiazine-4(3H)-one-3-ate-2,2-dioxide.
Effect on body weight
Acesulfame potassium provides a sweet taste with no caloric value. There is no high-quality evidence that using acesulfame potassium as a sweetener affects body weight or body mass index (BMI).[12][13][14]
Discovery
Acesulfame potassium was developed after the accidental discovery of a similar compound (5,6-dimethyl-1,2,3-oxathiazin-4(3H)-one 2,2-dioxide) in 1967 by Karl Clauss and Harald Jensen at Hoechst AG.[15][16] After accidentally dipping his fingers into the chemicals with which he was working, Clauss licked them to pick up a piece of paper.[17] Clauss is the inventor listed on a United States patent issued in 1975 to the assignee Hoechst Aktiengesellschaft for one process of manufacturing acesulfame potassium.[18] Subsequent research showed a number of compounds with the same basic ring structure had varying levels of sweetness. 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide had particularly favourable taste characteristics and was relatively easy to synthesize, so it was singled out for further research, and received its generic name (acesulfame-K) from the World Health Organization in 1978.[15] Acesulfame potassium first received approval for table top use in the United States in 1988.[10]
Safety
As with other artificial sweeteners, concern exists over the safety of acesulfame potassium. However, the United States Food and Drug Administration (FDA) has approved its general use. Critics say acesulfame potassium has not been studied adequately and may be carcinogenic,[19] although these claims have been dismissed by the European Food Safety Authority[20] and FDA.[21]
Environment Canada tested the water from the Grand River at 23 sites between its headwaters and where it flows into Lake Erie. The results suggest that acesulfame appears in far higher concentrations than saccharin or sucralose at the various test sites.[22]
Compendial status
References
- "acesulfame–K". Merriam-Webster. Merriam-Webster. Archived from the original on 10 March 2017. Retrieved 31 January 2017.
- "Current EU approved additives and their E Numbers". UK: Food Standards Agency. 2012-03-14. Archived from the original on 2013-07-19. Retrieved 2012-08-07.
- Clauss, K.; Jensen, H. (1973). "Oxathiazinone Dioxides - A New Group of Sweetening Agents". Angewandte Chemie International Edition. 12 (11): 869–876. doi:10.1002/anie.197308691.
- Ager, D. J.; Pantaleone, D. P.; Henderson, S. A.; Katritzky, A. R.; Prakash, I.; Walters, D. E. (1998). "Commercial, Synthetic Nonnutritive Sweeteners" (PDF). Angewandte Chemie International Edition. 37 (13–14): 1802–1817. doi:10.1002/(SICI)1521-3773(19980803)37:13/14<1802::AID-ANIE1802>3.0.CO;2-9. Archived from the original (PDF) on 2008-09-10.
- United States Patent 5,336,513 (expired in 2006)
- Deis RC (November 2006). "Customizing Sweetness Profiles" (PDF). Food Product Design. Archived from the original (PDF) on 11 August 2014. Retrieved 16 May 2018.
- Mullarney, M.; Hancock, B.; Carlson, G.; Ladipo, D.; Langdon, B. The powder flow and compact mechanical properties of sucrose and three high-intensity sweeteners used in chewable tablets. Int. J. Pharm. 2003, 257, 227–236.
- Findikli, Z.; Zeynep, F.; Sifa, T. Determination of the effects of some artificial sweeteners on human peripheral lymphocytes using the comet assay. Journal of toxicology and environmental health sciences 2014, 6, 147–153.
- "Home - WHO - Prequalification of Medicines Programme". Retrieved 2 March 2017.
- Whitehouse, C.; Boullata, J.; McCauley, L. The potential toxicity of artificial sweeteners. AAOHN J. 2008, 56, 251-9 quiz 260.
- Erika Engelhaupt (March 1, 2017). "Just How Much Pee Is In That Pool?". NPR. Archived from the original on March 1, 2017. Retrieved March 2, 2017.
- Miller PE, Perez V (September 2014). "Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies". The American Journal of Clinical Nutrition. 100 (3): 765–777. doi:10.3945/ajcn.113.082826. PMC 4135487. PMID 24944060.
- Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, Mann A, Jeyaraman MM, Reid AE, Fiander M, MacKay DS, McGavock J, Wicklow B, Zarychanski R (July 2017). "Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies". CMAJ. 189 (28): E929–E939. doi:10.1503/cmaj.161390. PMC 5515645. PMID 28716847.
- Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, Ness AR, Penfold C, Perry R, Putz P, Yeomans MR, Mela DJ (September 2015). "Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies". International Journal of Obesity. 40 (3): 381–94. doi:10.1038/ijo.2015.177. PMC 4786736. PMID 26365102.
- O'Brien-Nabors, L. (2001). Alternative Sweeteners. New York, NY: Marcel Dekker. p. 13. ISBN 978-0-8247-0437-7.
- Williams, R. J.; Goldberg, I. (1991). Biotechnology and Food Ingredients. New York: Van Nostrand Reinhold. ISBN 978-0-442-00272-5.
- Newton, D. E. (2007). Food Chemistry (New Chemistry). New York: Infobase Publishing. p. 69. ISBN 978-0-8160-5277-6. Archived from the original on 2016-03-05. Retrieved 2017-09-08.
- US 3917589, Clauss, K., "Process for the manufacture of 6-methyl-3,4-dihydro-1,2,3-oxathiazine-4-one-2,2-dioxide", issued 1975
- Karstadt, M. L. (2006). "Testing Needed for Acesulfame Potassium, an Artificial Sweetener". Environmental Health Perspectives. 114 (9): A516, author reply A516–7. doi:10.1289/ehp.114-a516a. PMC 1570055. PMID 16966071.
- Scientific Committee on Food (2000). "Opinion - Re-evaluation of acesulfame K with reference to the previous SCF opinion of 1991" (PDF). SCF/CS/ADD/EDUL/194 final. EU Commission. Archived from the original (PDF) on 2008-09-10. Retrieved 2007-10-04.
- Kroger, M.; Meister, K.; Kava, R. (2006). "Low-Calorie Sweeteners and Other Sugar Substitutes: A Review of the Safety Issues". Comprehensive Reviews in Food Science and Food Safety. 5 (2): 35–47. doi:10.1111/j.1541-4337.2006.tb00081.x.
- "Major Canadian river contains artificial sweeteners". Waterloo News. University of Waterloo. December 13, 2013. Archived from the original on October 21, 2014. Retrieved October 17, 2014.
- British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009" (PDF). Archived from the original (PDF) on 2009-04-11.
External links
| Wikimedia Commons has media related to Acesulfame potassium. |
- Joint FAO/WHO Expert Committee on Food Additives evaluation monograph of Acesulfame Potassium
- FDA approval of Acesulfame Potassium
- FDA approval of Acesulfame Potassium as a General Purpose Sweetener in Food
- Elmhurst College, Illinois Virtual ChemBook Acesulfame K
- Discovery News Sweeteners Linger in Groundwater
