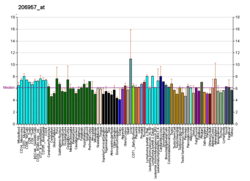
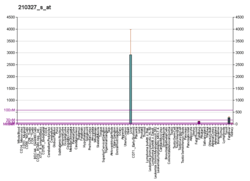
AGXT
Serine—pyruvate aminotransferase is an enzyme that in humans is encoded by the AGXT gene.[5][6][7]
This gene is expressed only in the liver and the encoded protein is localized mostly in the peroxisomes, where it is involved in glyoxylate detoxification. Mutations in this gene, some of which alter subcellular targeting, have been associated with type I primary hyperoxaluria.[7]
See also
References
- GRCh38: Ensembl release 89: ENSG00000172482 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000026272 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Nishiyama K, Funai T, Katafuchi R, Hattori F, Onoyama K, Ichiyama A (Jul 1991). "Primary hyperoxaluria type I due to a point mutation of T to C in the coding region of the serine:pyruvate aminotransferase gene". Biochem Biophys Res Commun. 176 (3): 1093–9. doi:10.1016/0006-291X(91)90396-O. PMID 2039493.
- Purdue PE, Lumb MJ, Fox M, Griffo G, Hamon-Benais C, Povey S, Danpure CJ (Jul 1991). "Characterization and chromosomal mapping of a genomic clone encoding human alanine:glyoxylate aminotransferase". Genomics. 10 (1): 34–42. doi:10.1016/0888-7543(91)90481-S. PMID 2045108.
- "Entrez Gene: AGXT alanine-glyoxylate aminotransferase (oxalosis I; hyperoxaluria I; glycolicaciduria; serine-pyruvate aminotransferase)".
External links
- GeneReviews/NIH/NCBI/UW entry on Primary Hyperoxaluria Type 1
- Human AGT genome location and AGT gene details page in the UCSC Genome Browser.
- Human AGXT genome location and AGXT gene details page in the UCSC Genome Browser.
Further reading
- Danpure CJ (1993). "Primary hyperoxaluria type 1 and peroxisome-to-mitochondrion mistargeting of alanine:glyoxylate aminotransferase". Biochimie. 75 (3–4): 309–15. doi:10.1016/0300-9084(93)90091-6. PMID 8507692.
- Danpure CJ (2005). "Molecular etiology of primary hyperoxaluria type 1: new directions for treatment". Am. J. Nephrol. 25 (3): 303–10. doi:10.1159/000086362. PMID 15961951.
- Minatogawa Y, Tone S, Allsop J, et al. (1993). "A serine-to-phenylalanine substitution leads to loss of alanine:glyoxylate aminotransferase catalytic activity and immunoreactivity in a patient with primary hyperoxaluria type 1". Hum. Mol. Genet. 1 (8): 643–4. doi:10.1093/hmg/1.8.643. PMID 1301173.
- Purdue PE, Lumb MJ, Allsop J, et al. (1992). "A glycine-to-glutamate substitution abolishes alanine:glyoxylate aminotransferase catalytic activity in a subset of patients with primary hyperoxaluria type 1". Genomics. 13 (1): 215–8. doi:10.1016/0888-7543(92)90225-H. PMID 1349575.
- Purdue PE, Takada Y, Danpure CJ (1991). "Identification of mutations associated with peroxisome-to-mitochondrion mistargeting of alanine/glyoxylate aminotransferase in primary hyperoxaluria type 1". J. Cell Biol. 111 (6 Pt 1): 2341–51. doi:10.1083/jcb.111.6.2341. PMC 2116406. PMID 1703535.
- Purdue PE, Allsop J, Isaya G, et al. (1992). "Mistargeting of peroxisomal L-alanine:glyoxylate aminotransferase to mitochondria in primary hyperoxaluria patients depends upon activation of a cryptic mitochondrial targeting sequence by a point mutation". Proc. Natl. Acad. Sci. U.S.A. 88 (23): 10900–4. doi:10.1073/pnas.88.23.10900. PMC 53039. PMID 1961759.
- Nishiyama K, Berstein G, Oda T, Ichiyama A (1991). "Cloning and nucleotide sequence of cDNA encoding human liver serine-pyruvate aminotransferase". Eur. J. Biochem. 194 (1): 9–18. doi:10.1111/j.1432-1033.1990.tb19420.x. hdl:10271/960. PMID 2253628.
- Takada Y, Kaneko N, Esumi H, et al. (1990). "Human peroxisomal L-alanine: glyoxylate aminotransferase. Evolutionary loss of a mitochondrial targeting signal by point mutation of the initiation codon". Biochem. J. 268 (2): 517–20. doi:10.1042/bj2680517. PMC 1131464. PMID 2363689.
- Danpure CJ, Jennings PR (1986). "Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I". FEBS Lett. 201 (1): 20–4. doi:10.1016/0014-5793(86)80563-4. PMID 3709805.
- Danpure CJ, Fryer P, Jennings PR, et al. (1995). "Evolution of alanine:glyoxylate aminotransferase 1 peroxisomal and mitochondrial targeting. A survey of its subcellular distribution in the livers of various representatives of the classes Mammalia, Aves and Amphibia". Eur. J. Cell Biol. 64 (2): 295–313. PMID 7813517.
- Danpure CJ, Purdue PE, Fryer P, et al. (1993). "Enzymological and mutational analysis of a complex primary hyperoxaluria type 1 phenotype involving alanine:glyoxylate aminotransferase peroxisome-to-mitochondrion mistargeting and intraperoxisomal aggregation". Am. J. Hum. Genet. 53 (2): 417–32. PMC 1682352. PMID 8101040.
- Minatogawa Y, Kawai C, Hatada S, Sato M (1997). "Liver specific kynurenine(alanine):glyoxylate aminotransferase was expressed in kidney cell line". Adv. Exp. Med. Biol. 398: 471–6. doi:10.1007/978-1-4613-0381-7_73. PMID 8906307.
- von Schnakenburg C, Rumsby G (1997). "Primary hyperoxaluria type 1: a cluster of new mutations in exon 7 of the AGXT gene". J. Med. Genet. 34 (6): 489–92. doi:10.1136/jmg.34.6.489. PMC 1050973. PMID 9192270.
- Amoroso A, Pirulli D, Puzzer D, et al. (1999). "Gene symbol: AGXT. Disease: primary hyperoxaluria type I". Hum. Genet. 104 (5): 441. doi:10.1007/s004390050984. PMID 10394939.
- Pirulli D, Puzzer D, Ferri L, et al. (1999). "Molecular analysis of hyperoxaluria type 1 in Italian patients reveals eight new mutations in the alanine: glyoxylate aminotransferase gene". Hum. Genet. 104 (6): 523–5. doi:10.1007/s004390050998. PMID 10453743.
- Basmaison O, Rolland MO, Cochat P, Bozon D (2000). "Identification of 5 novel mutations in the AGXT gene". Hum. Mutat. 15 (6): 577. doi:10.1002/1098-1004(200006)15:6<577::AID-HUMU9>3.0.CO;2-#. PMID 10862087.
- Lumb MJ, Danpure CJ (2000). "Functional synergism between the most common polymorphism in human alanine:glyoxylate aminotransferase and four of the most common disease-causing mutations". J. Biol. Chem. 275 (46): 36415–22. doi:10.1074/jbc.M006693200. PMID 10960483.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.