Alcohol by volume
Alcohol by volume (abbreviated as ABV, abv, or alc/vol) is a standard measure of how much alcohol (ethanol) is contained in a given volume of an alcoholic beverage (expressed as a volume percent).[1][2][3] It is defined as the number of millilitres (mL) of pure ethanol present in 100 mL (3.4 fl. oz) of solution at 20 °C (68 °F). The number of millilitres of pure ethanol is the mass of the ethanol divided by its density at 20 °C, which is 0.78924 g/mL (105.3 fl oz/gallon). The ABV standard is used worldwide. The International Organization of Legal Metrology has tables of density of water–ethanol mixtures at different concentrations and temperatures.

In some countries, e.g. France, alcohol by volume is often referred to as degrees Gay-Lussac (after the French chemist Joseph Louis Gay-Lussac),[4] although there is a slight difference since the Gay-Lussac convention uses the International Standard Atmosphere value for temperature, 15 °C (59 °F).
Volume change
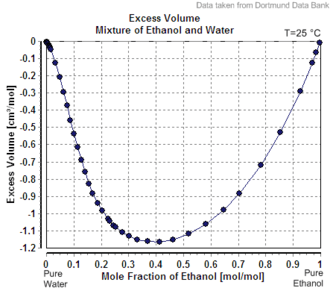
Mixing two solutions of alcohol of different strengths usually causes a change in volume. Mixing pure water with a solution less than 24% by mass causes a slight increase in total volume, whereas the mixing of two solutions above 24% causes a decrease in volume.[lower-alpha 1] The phenomenon of volume changes due to mixing dissimilar solutions is called "partial molar volume". Water and ethanol are both polar solvents. When water is added to ethanol, the smaller water molecules are attracted to the ethanol's hydroxyl group, and each molecule alters the polarity field of the other. The attraction allows closer spacing between molecules than is usually found in non-polar mixtures.
Thus, ABV is not the same as volume fraction expressed as a percentage. Volume fraction, which is widely used in chemistry (commonly denoted as v/v), is defined as the volume of a particular component divided by the sum of all components in the mixture when they are measured separately. For example, to make 100mL of 50% ABV ethanol solution, water would be added to 50mL of ethanol to make up exactly 100mL. Whereas to make a 50% v/v ethanol solution 50 mL of ethanol and 50 mL of water could be mixed but the resulting volume of solution will measure less than 100 mL due to the change of volume on mixing, and will contain a higher concentration of ethanol.[5] The difference is not large, with the maximum difference being less than 2.5%, and less than 0.5% difference for concentrations under 20%.
Typical levels
Details about typical amounts of alcohol contained in various beverages can be found in the articles about them.
| Drink | Typical ABV |
|---|---|
| Fruit juice (naturally occurring) | 0–0.09% |
| Low-alcohol beer | 0.05–1.2% |
| Kvass | 0.05–1.5% |
| Kefir | 0.2–2.0% |
| Kombucha | 0.5–1.5% |
| Boza | 1.0% |
| Chicha | 1.0–11% (usually 1–6%) |
| Tubâ | 2.0–4.0% |
| Beer | 2.0–12% (usually 4–6%) |
| Cider | 2.0–12% (usually 4–8%) |
| Alcopops | 4.0–17.5% |
| Malt liquor | 5.0%+ |
| Makgeolli | 6.5–7% |
| Kuchikamizake | 7%[6] |
| Barley wine (strong ale) | 8–15% |
| Mead | 8–16% |
| Wine | 5.5–16% (most often 12.5–14.5%)[7][8] |
| Bahalina | 10–13% |
| Basi | 10–16% |
| Bignay wine | 12–13% |
| Duhat wine | 12–13% |
| Tapuy | 14–19% |
| Kilju | 15–17% |
| Dessert wine | 14–25% |
| Sake | 15% (or 18–20% if not diluted prior to bottling) |
| Liqueurs | 15–55% |
| Fortified wine | 15.5–20%[9] (in the European Union, 18–22%) |
| Soju | 14–45% (usually 17%) |
| Shochu | 25–45% (usually 25%) |
| Rượu đế | 27–45% (usually 35% – except Ruou tam – 40–45%) |
| Bitters | 28–45% |
| Applejack | 30–40% |
| Pisco | 30–48% |
| Mezcal, Tequila | 32–60% (usually 40%) |
| Vodka | 35–95% (usually 40%, minimum of 37.5% in the European Union) |
| Rum | 37.5–80% (usually 40%) |
| Brandy | 35–60% (usually 40%) |
| Grappa | 37.5–60% |
| Ouzo | 37.5%+ |
| Gin | 37.5–50% |
| Pálinka | 37.5–86% (usually 52%) |
| Cachaça | 38–48% |
| Sotol | 38–60% |
| Stroh | 38–80% |
| Fernet | 39–45% |
| Lambanog | 40–45% |
| Nalewka | 40–45% |
| Tsipouro | 40–45% |
| Scotch whisky | 40–63.5% |
| Whisky | 40–68% (usually 40%, 43% or 46%) |
| Baijiu | 40–65% |
| Rakija | 40–65% |
| Chacha | 40–70% |
| Răchie (Central/Southeast European drink) | 42–86% |
| Maotai | 43–53% |
| Absinthe | 45–89.9% |
| Țuică (Romanian drink) | 30–65% (usually 35–55%) |
| Arak | 60–65% |
| Oghi | 60–75% |
| Poitín | 60–95% |
| Centerbe (herb liqueur) | 70% |
| Neutral grain spirit | 85–95% |
| Cocoroco | 93–96% |
| Rectified spirit | 95% up to a practical limit of 97.2% |
Practical estimation of alcohol content
During the production of wine and beer, yeast is added to a sugary solution. During fermentation, the yeasts consume the sugars and produce alcohol. The density of sugar in water is greater than the density of alcohol in water. A hydrometer is used to measure the change in specific gravity (SG) of the solution before and after fermentation. The volume of alcohol in the solution can then be estimated. There are a number of empirical formulae which brewers and winemakers use to estimate the alcohol content of the liquor made.
Specific gravity is the density of a liquid relative to that of water, i.e if the density of the liquid is 1.05 times that of water it has a specific gravity of 1.05. In UK brewing usage it is customary to regard the reference value for water to be 1000, so the specific gravity of the same example beer would be quoted as 1050. The formulas here assume that the former definition is used for specific gravity.
Other methods of specifying alcohol content
Alcohol proof
Another way of specifying the amount of alcohol content is alcohol proof, which in the United States is twice the alcohol-by-volume (ABV) number. This may lead to confusion over similar products bought in varying regions that have different names on country specific labels. For example, Stroh rum that is 80% ABV is advertised and labeled as Stroh 80 when sold in Europe, but is named Stroh 160 when sold in the United States.
In the United Kingdom proof is 1.75 times the number (expressed as a percentage)[13][10]. For example, 40% abv is 80 proof in the US and 70 proof in the UK. However, since 1980, alcohol proof in the UK has been replaced by ABV as a measure of alcohol content, avoiding confusion between the UK and US proof standards.
Alcohol by weight
In the United States and India, a few states regulate and tax alcoholic beverages according to alcohol by weight (ABW), expressed as a percentage of total mass. Some brewers print the ABW (rather than the ABV) on beer containers, particularly on low-point versions of popular domestic beer brands. The ABV value of a beverage is always higher than the ABW.
Because ABW measures the proportion of the drink's mass which is alcohol, while ABV is the proportion of the drink's volume which is alcohol, the two values are in the same proportion as the drink's density is with the density of alcohol. Therefore one can use the following equation to convert between ABV and ABW:
At relatively low ABV, the alcohol percentage by weight is about 4/5 of the ABV (e.g., 3.2% ABW is about 4% ABV).[14] However, because of the miscibility of alcohol and water, the conversion factor is not constant but rather depends upon the concentration of alcohol. At 0% and 100% ABV is equal to ABW, but at values in between ABV is always higher, up to ~13% higher around 60% ABV.
See also
- Apparent molar property
- Excess molar quantity
- Standard drink
- Unit of alcohol
- Volume fraction
Notes
- See data in the CRC Handbook of Chemistry and Physics, 49th edition, pp. D-151 and D-152. Mixing a solution above 24% with a solution below 24% may cause an increase or a decrease, depending on the details.
References
- "Lafayette Brewing Co". www.lafayettebrewingco.com. Archived from the original on 2012-02-19. Retrieved 2012-02-04.
- "Glossary of whisky and distillation". www.celtic-whisky.com. Archived from the original on 2012-02-12. Retrieved 2012-02-04.
- "English Ales Brewery Monterey British Brewing Glossary". www.englishalesbrewery.com. Archived from the original on 2012-02-19. Retrieved 2012-02-04.
- "Joseph Louis Gay-Lussac (1778–1850)". chemistry.about.com. Retrieved 2008-07-05.
- "Density ρ of Ethanol-Water Mixtures at the Temperature in °C Indicated by Superscript". CRC Handbook of Chemistry and Physics. Retrieved 2019-12-13.
This source gives density data for ethanol:water mixes by %weight ethanol in 5% increments and against temperature including at 25 °C, used here. It can be calculated from this table that at 25 °C, 45 g of ethanol has volume 57.3 ml, 55 g of water has volume 55.2 ml; these sum to 112.5 ml. When mixed they have volume 108.6 ml. - "Brewing (and Chewing) the Origins of Sake". Boston Sake. April 2, 2012.
- Robinson 2006, p. 10.
- "Wine: From the Lightest to the Strongest". Wine Folly. 2015-11-23. Retrieved 2019-06-20.
- Robinson 2006, p. 279.
- Berry 1998.
- "Get to Know Your Alcohol (By Volume)". BeerAdvocate.com. 18 June 2003. Archived from the original on 3 July 2014.
- Peros, Roko (7 May 2010). "Calculate Percent Alcohol in Beer". BrewMoreBeer.com.
- Regan 2003.
- "Alcohol Content In Beer". www.realbeer.com. Archived from the original on 4 July 2008. Retrieved 5 July 2008.
Bibliography
- Hehner, Otto (1880). Alcohol Tables: giving for all specific gravities, from 1.0000 to 0.7938, the percentages of absolute alcohol, by weight and volume. London: J & A Churchill. ASIN B0008B5HOU.
- Berry, C. J. J. (1998). First Steps in Winemaking. Nexus Special Interests. ISBN 978-1-85486-139-9.CS1 maint: ref=harv (link)
- Regan, Gary (2003). The Joy of Mixology. Clarkson Potter. ISBN 978-0-609-60884-5.CS1 maint: ref=harv (link)
- Robinson, Jancis (2006). The Oxford Companion to Wine (3rd ed.). Oxford: OUP. ISBN 978-0-19-860990-2.CS1 maint: ref=harv (link)
External links
- "How do brewers measure the alcohol in beer?". HowStuffWorks. 12 December 2000.
- Jayes, Wayne. "Alcohol Strength and Density". sugartech.co.za. The Sugar Engineers.